Method for synthesizing second generation Grubbs catalyst
A synthesis method and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., to achieve good overall yield, simple, safe and easy-to-control operation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
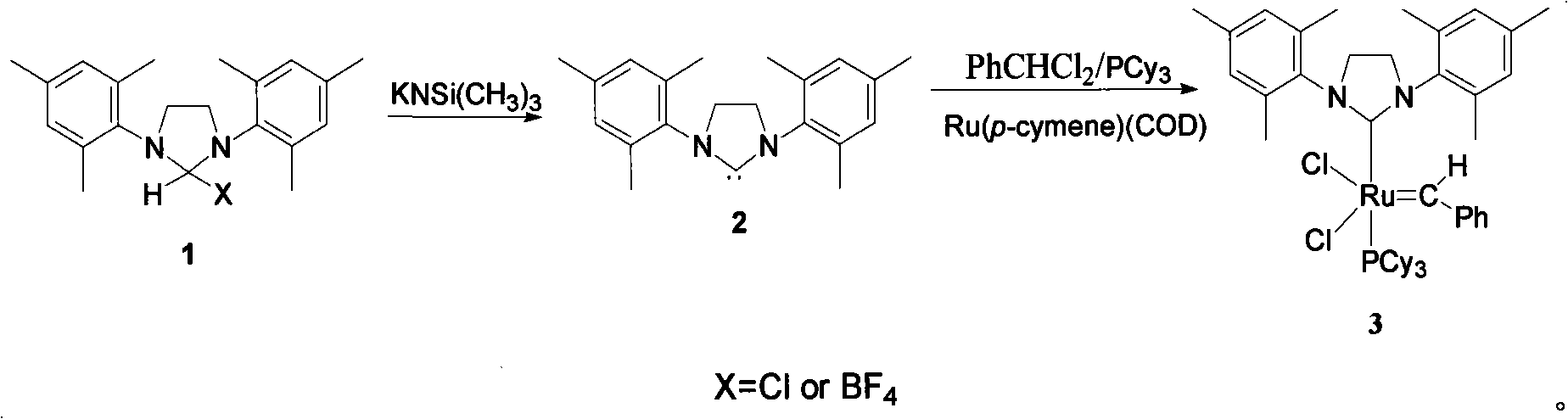
[0020] Step 1 Synthesis of compound H 2 IMes 2
[0021] Add H to the reaction flask 2 Imes(H)(Cl)(0.40g, 1.16mmol), THF(10mL), dissolved and added KNSi(CH 3 ) 3 (0.53mL, 1.16mmol), stirred at room temperature for 0.5h, filtered to remove the residue, and the filtrate was dried, and after vacuum drying, compound H 2 IMes, 83% yield.
[0022] Step 2 synthesizes the second generation Grubbs catalyst (H 2 IMes) (PCy 3 )(Cl) 2 Ru=CHPh3
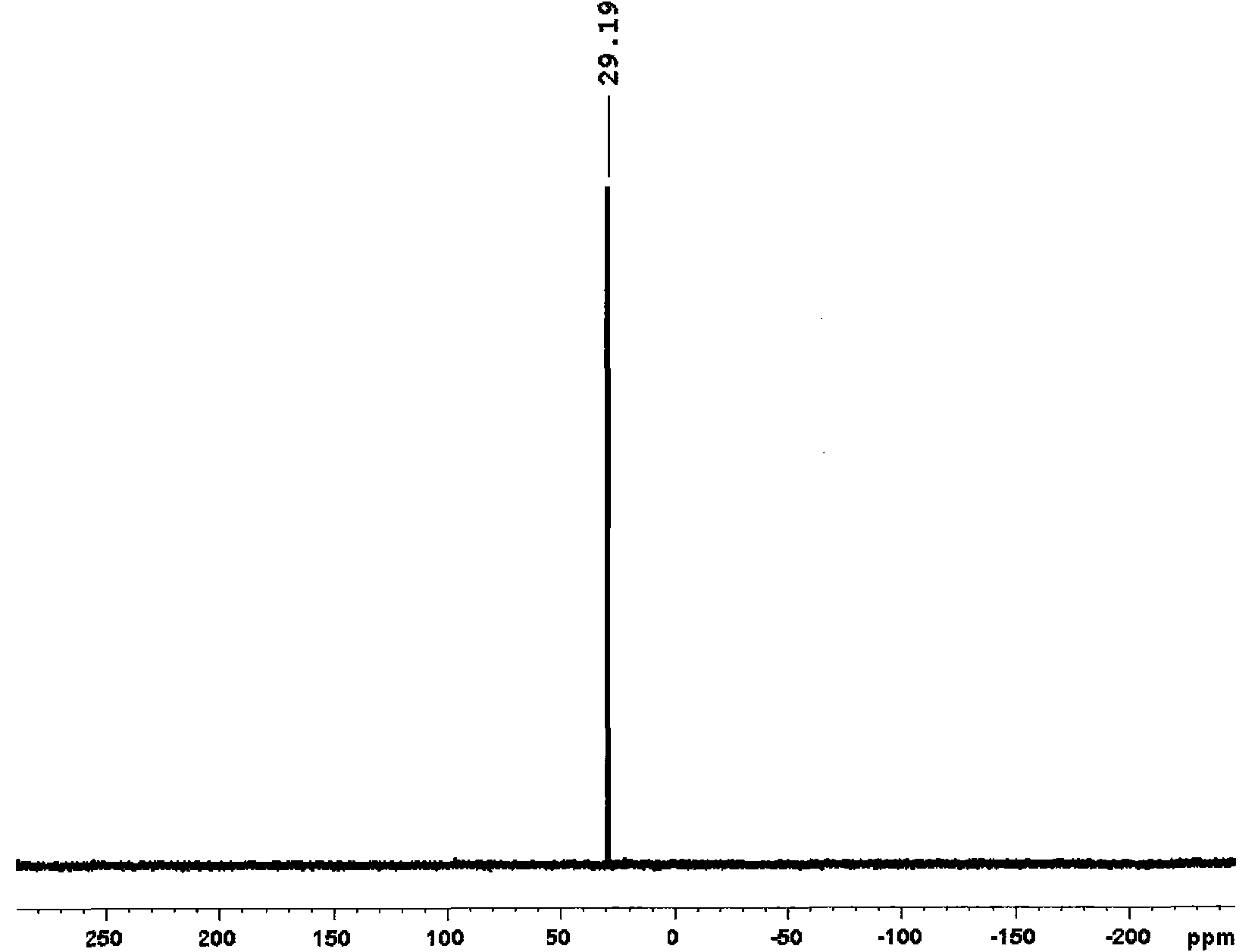
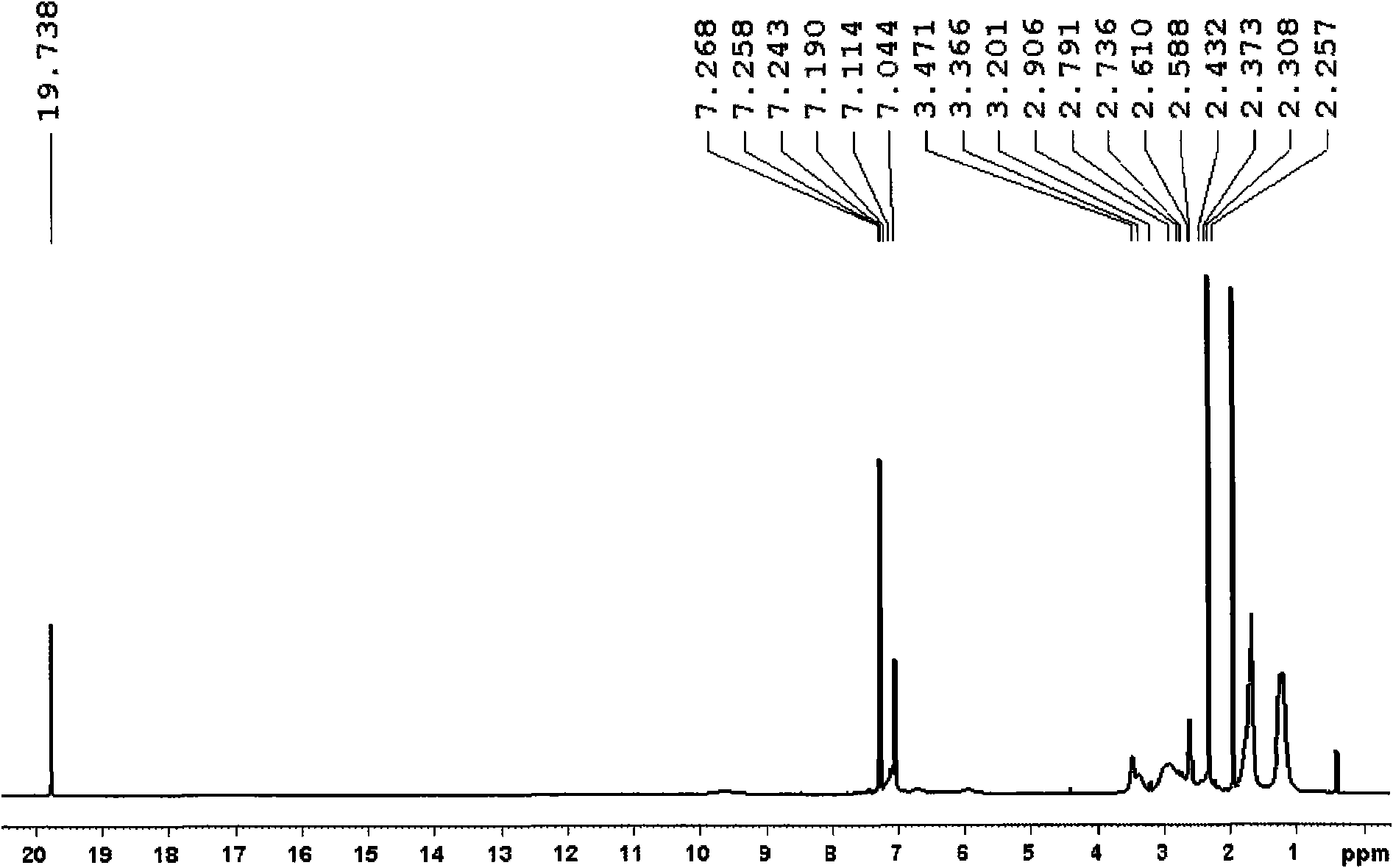
[0023] Add compound H to the reaction vial 2 IMes 2 (181mg, 0.59mmol), Ru(p-cymene) (COD) (200mg, 0.59mmol), PCy 3 (165mg, 0.59mmol) and toluene (10mL), dissolved and added PhCHCl 2 (0.10mL, 0.79mmol), stirred and reacted at room temperature for 30h, concentrated the solution under reduced pressure to about 1mL, added 10mL of methanol, precipitated a purple-brown solid, filtered it with a sand core, washed the solid with 3×3mL methanol, and dried in vacuo to obtain the compound (H 2 IMes) (PCy 3 )(Cl) 2 Ru=CHPh3, yield 50%. figure 1 ...
Embodiment 2
[0025] Step 1 Synthesis of compound H 2 IMes 2
[0026] Compound H 2 The operation of IMes 2 is identical with the operation in embodiment 1, reactant H 2 IMes(H)(Cl) changed to H 2 Imes(H)(BF 4 ), yield 84%.
[0027] Step 2 synthesizes the second generation Grubbs catalyst (H 2 IMes) (PCy 3 )(Cl) 2 Ru=CHPh3
[0028] Compound (H 2 IMes) (PCy 3 )(Cl) 2 The synthesis steps of Ru=CHPh 3 are the same as in Example 1.
Embodiment 3
[0030] Step 1 Synthesis of compound H 2 IMes 2
[0031] Compound H 2 The operation of IMes 2 was the same as that in Example 1, the solvent was changed to hexane, stirred at room temperature for 2 h, and the yield was 85%.
[0032] Step 2 synthesizes the second generation Grubbs catalyst (H 2 IMes) (PCy 3 )(Cl) 2 Ru=CHPh3
[0033] Compound (H 2 IMes) (PCy 3 )(Cl) 2 The synthesis steps of Ru=CHPh 3 are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com