Medical composition containing rebamipide
A technology of rebamipide and composition, applied in the direction of drug combination, medical preparations containing active ingredients, medical preparations without active ingredients, etc., can solve the problem of reducing the stability of drug ingredients and unsatisfactory suppression of bitter taste , increasing bitterness, spicy and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086]Utilize vertical granulator, the mixture of 1010g rebamipide, 1845g lactose, 510g cornstarch, 140g crystalline cellulose, 680g dextrin, 150g sodium carboxymethyl starch and 90g croscarmellose sodium and A mixed solution of 20 g of L-arginine, 838 g of purified water, and 140 g of polysorbate 80 was kneaded. The resulting mixture was granulated using an extrusion granulator (TDG-80, manufactured by Fuji Trading Co.) equipped with a screen with a pore size of 0.6 mm, and then prepared using a spherical granulator (QJ-400, manufactured by Fuji Trading Co.) spherical. The obtained particles were dried, and particles having a particle diameter of 0.355 to 1 mm were collected as core particles.
[0087] Separately, citrate fatty acid glyceride (SunSoft 621G, manufactured by Sun Chemical, 60 g) was added to a mixture of 144 g of ethanol and 36 g of water, and dissolved by heating. Add D-mannitol (60g), then stir to suspend it to obtain a coating solution.
[0088] Nuclei gra...
Embodiment 2
[0095] Using a vertical granulator, 60.6g rebamipide, 110.7g lactose, 30.6g corn starch, 8.4g crystalline cellulose, 36.0g dextrin, 9.0g sodium carboxymethyl starch and 5.4g cross-linked carboxymethyl The mixture of sodium cellulose and 6 g of L-arginine, 50.3 g of purified water and 8.4 g of polysorbate 80 were kneaded. The same operation was repeated 3 times, and the kneaded material obtained was granulated using an extrusion granulator (TDG-80, manufactured by Fuji Trading Co., Ltd.) equipped with a screen with an aperture of 0.6 mm, and then granulated using a spherical granulator (QJ -400, manufactured by Fuji Trading Co., Ltd.) into a spherical shape. The obtained particles were dried, and particles having a particle diameter of 0.355 to 1 mm were collected as core particles. Coated granules were produced in the same manner as in Example 1.
Embodiment 3~7
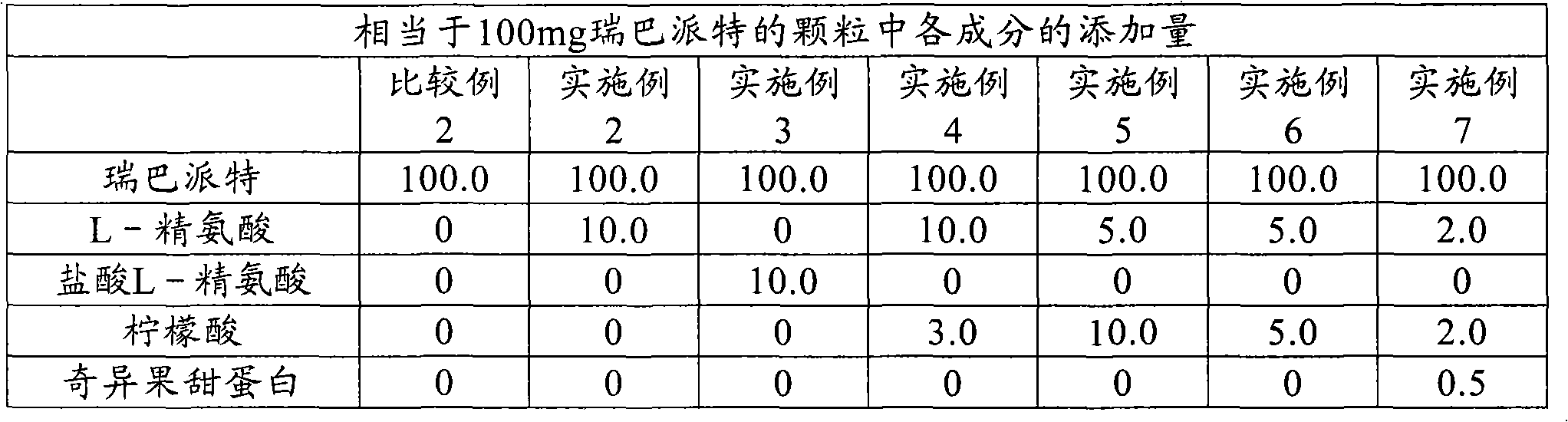
[0099] Produced in the same manner as in Example 2, except that rebamipide, L-arginine (or L-arginine hydrochloride), citric acid, and thaumatin were used in the amounts shown in Table 1 below. The coated granules of Examples 3-7. In addition, citric acid and L-arginine hydrochloride were added to the liquid mixture in the same manner as L-arginine. Thaumatin is not added to the mixture, but added to rebamipide, lactose, etc. By controlling the amount of dextrin, the weight changes caused by the changes in the amounts of L-arginine, L-arginine hydrochloride, citric acid, and thaumatin were adjusted so that the total weight of the pharmaceutical preparation was the same in each case.
[0100] [Table 1]
[0101]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com