Method for preparing cadmium sulfide-titanium dioxide nano-tube composite catalyst
A nanotube composite and catalyst technology is applied in physical/chemical process catalysts, chemical instruments and methods, hydrogen production, etc. simple method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Step 1, take 0.40g anatase TiO 2 Nanoparticles, placed in a polytetrafluoroethylene reactor, add 100ml deionized water, stir until evenly mixed;
[0027] Step 2, add 2.76ml 0.01molL to the polytetrafluoroethylene reaction kettle in turn -1 CdCl 2 2.5H 2 Aqueous solution of O and 2.76ml of 0.01molL -1 Na 2 S9H 2 O aqueous solution, mixed, then added 40g NaOH, and ultrasonically oscillated for 30min;
[0028] Step 3, place the polytetrafluoroethylene reactor in a microwave reactor with a reflux device, microwave the polytetrafluoroethylene reactor, the microwave emission power is 300w, and the microwave heating time is 120min; then let it stand for 6 hours, use Wash the product with deionized water until the pH of the washing solution is 7, filter it with suction, and dry it in vacuum at 80°C to obtain CdS-TiO 2 Nanotube Composite Catalysts.
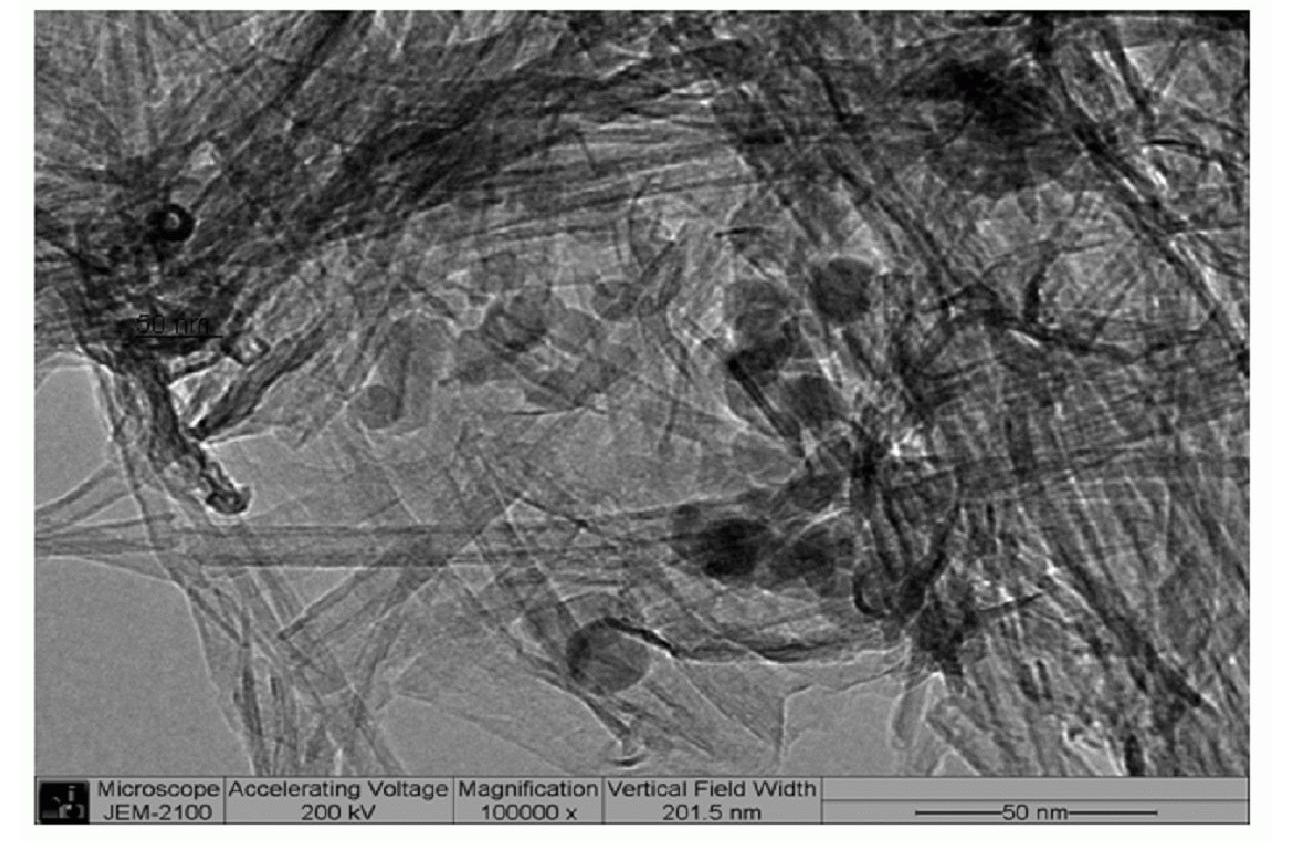
[0029] Implementation effect of this embodiment: figure 1 CdS-TiO prepared for this example 2 Photos of nanotube catalys...
Embodiment 2
[0031] Step 1, take 0.10g anatase TiO 2 Nanoparticles, placed in a polytetrafluoroethylene reactor, add 100ml deionized water, stir until evenly mixed;
[0032] Step 2, add 5.52ml 0.01molL to the polytetrafluoroethylene reaction kettle in turn -1 CdCl 2 2.5H2 Aqueous solution of O and 5.52ml of 0.01molL -1 Na 2 S9H 2 O aqueous solution, mixed, then added 40g NaOH, and ultrasonically oscillated for 30min;
[0033] Step 3, place the polytetrafluoroethylene reactor in a microwave reactor with a reflux device, microwave the polytetrafluoroethylene reactor, the microwave emission power is 300w, and the microwave heating time is 120min; then let it stand for 6 hours, use Wash the product with deionized water until the pH of the washing solution is 7, filter it with suction, and dry it in vacuum at 80°C to obtain CdS-TiO 2 Nanotube Composite Catalysts.
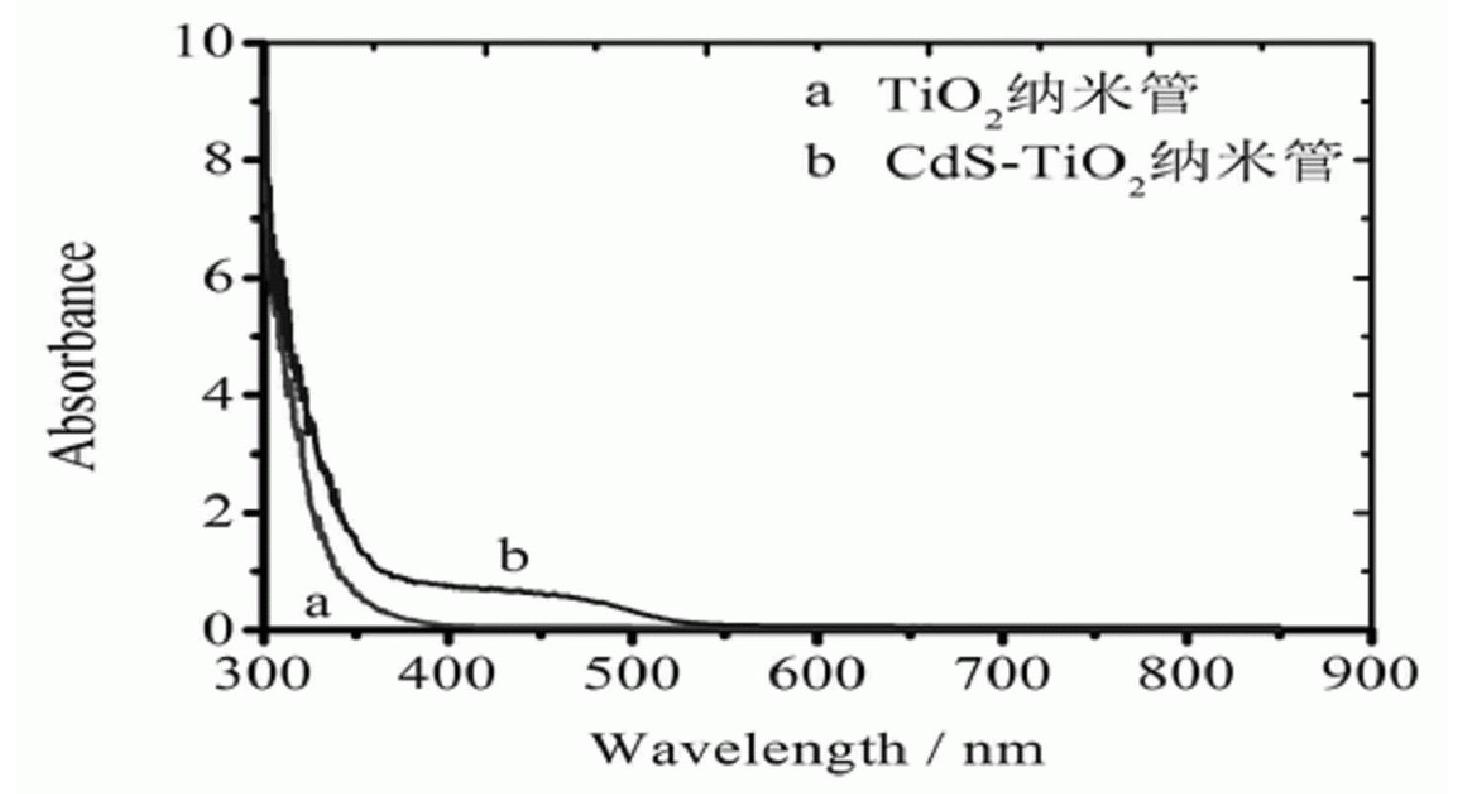
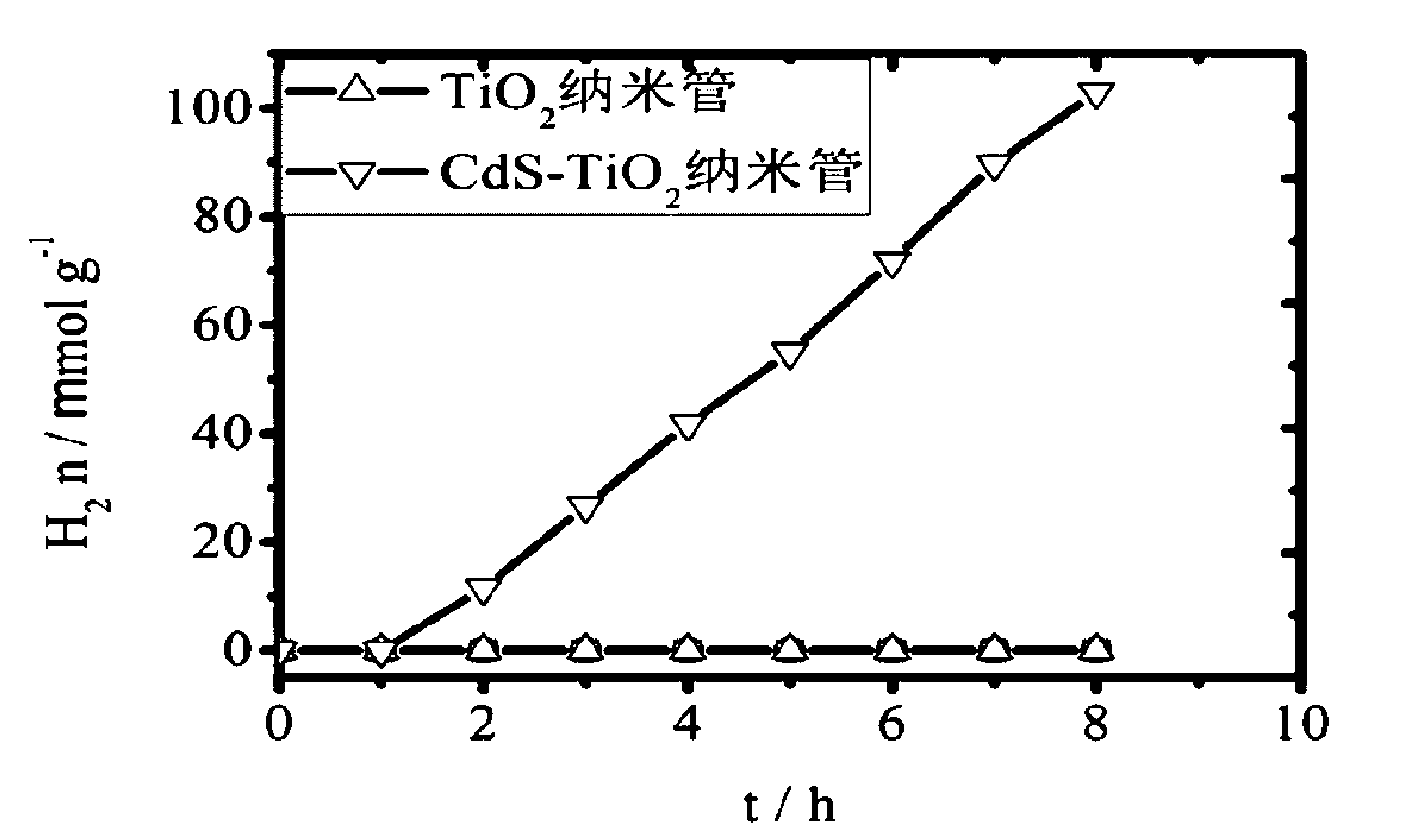
[0034] Implementation effect of this example: CdS-TiO prepared in this example 2 Catalytic properties of nanotube composite...
Embodiment 3
[0036] Step 1, take 0.20g anatase TiO 2 Nanoparticles, placed in a polytetrafluoroethylene reactor, add 100ml deionized water, stir until evenly mixed;
[0037] Step 2, add 11.04ml 0.01molL to the polytetrafluoroethylene reaction kettle in turn -1 CdCl 2 2.5H 2 O in water and 11.04ml of 0.01molL -1 Na 2 S9H 2 O aqueous solution, mixed, then added 40g NaOH, and ultrasonically oscillated for 30min;
[0038] Step 3, place the polytetrafluoroethylene reactor in a microwave reactor with a reflux device, microwave the polytetrafluoroethylene reactor, the microwave emission power is 300w, and the microwave heating time is 120min; then let it stand for 6 hours, use Wash the product with deionized water until the pH of the washing solution is 7, filter it with suction, and dry it in vacuum at 80°C to obtain CdS-TiO 2 Nanotube Composite Catalysts.
[0039] Implementation effect of this example: CdS-TiO prepared in this example 2 Catalytic properties of nanotube composite cataly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com