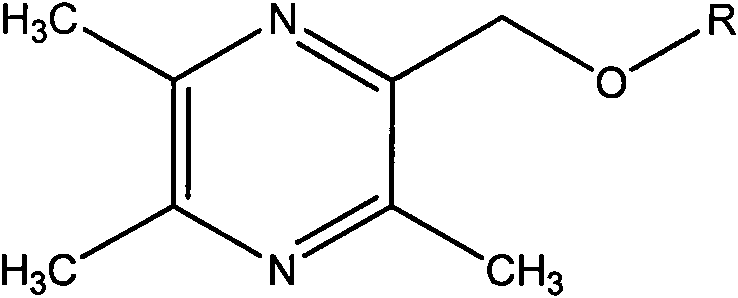
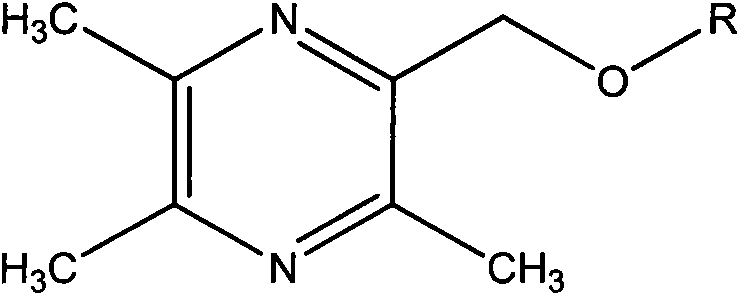
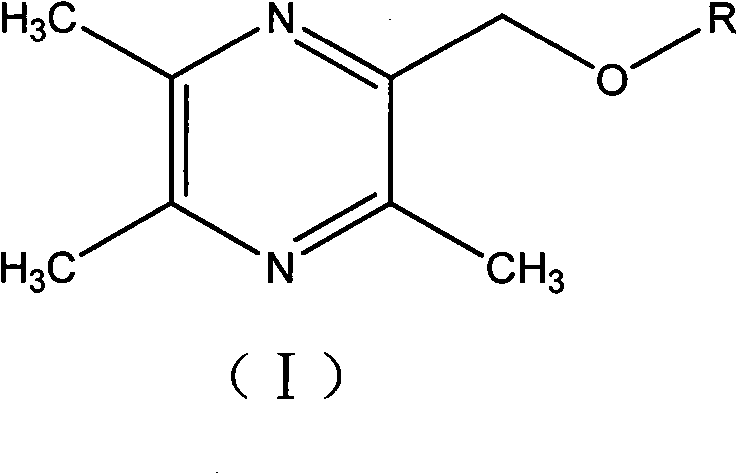
2-hydroxymethyl-3,5,6-trimethylpyrazine derivatives, preparation methods and application thereof
A technology of trimethylpyrazine and hydroxymethyl, applied in the field of 2-hydroxymethyl-3,5,6-trimethylpyrazine derivatives, its preparation and application, can solve unsatisfactory curative effect, Low bioavailability, short half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Example 1: (3,5,6-trimethylpyrazin-2-yl)methanol nitrate (HK-001)
[0135]
[0136] In the reaction bottle, add 2-hydroxymethyl-3,5,6-trimethylpyrazine (A) 15.2g, 60ml acetic acid, 10ml acetic anhydride, under stirring at room temperature, dropwise add 7.5g fuming nitric acid, in 20 The dropwise addition was completed within 1 minute, and then the reaction was incubated for 16 hours. TLC detected that the reaction was complete, and the reaction was stopped. The reaction solution was added to 200 g of crushed ice with stirring, and 120 ml of ethyl acetate was added, and the ethyl acetate layer was separated and dried over anhydrous magnesium sulfate. Ethyl acetate was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography (dichloromethane / ethyl acetate, 9:1) to obtain (3,5,6-trimethylpyrazin-2-yl)methanol nitrate 9.6g. HPLC: 99.1%, elemental analysis: (theoretical value / measured value), C (48.73 / 48.62), H (5.62 / 5.55), N (...
Embodiment 2
[0137] Example 2: (3,5,6-trimethylpyrazin-2-yl)methanol 4-((nitroxyl)methyl)benzoate (HK-002)
[0138]
[0139] a. In the reaction flask, add 7.6g of 2-hydroxymethyl-3,5,6-trimethylpyrazine (A), 80ml of dichloromethane, 8.0g of triethylamine, and stir at 15°C-20°C Add 9.5g of p-chloromethylbenzoyl chloride (B), and then react at 30° C. for 3 hours. TLC monitoring, the reaction is over, stop the reaction. Add 50ml of water, stir and let stand, separate the dichloromethane layer, and dry over anhydrous magnesium sulfate. Dichloromethane was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography (n-hexane / ethyl acetate, 9:2) to obtain 9.8 g of p-chloromethylbenzoic acid (2-hydroxymethyl-3,5,6- trimethylpyrazine) ester (C). HPLC: 98.11%, ESI-MS: 303.1 (M-H).
[0140] b. In the reaction flask, add 6.0 g of p-chloromethylbenzoic acid (2-hydroxymethyl-3,5,6-trimethylpyrazine) ester (C), 120 ml of acetonitrile, and heat to 65° C. und...
Embodiment 3
[0141] Example 3: 3-methyl-1,2,5-oxadiazole-2-oxide-4-carboxylic acid (3,5,6-trimethylpyrazin-2-yl)methanol ester (HK-003 )
[0142]
[0143] In the reaction flask, add 7.6g of 2-hydroxymethyl-3,5,6-trimethylpyrazine (A), 160ml of dichloromethane, 6.5g of pyridine, and add 8.9g of 3-methyl- 1,2,5-oxadiazole-2-oxide-4-formyl chloride (D), and then reacted at room temperature for 4.5 hours. TLC monitoring, the reaction is over, stop the reaction. Add 80ml of water, stir and let it stand, separate the dichloromethane layer, evaporate the dichloromethane under reduced pressure, and purify the residue by silica gel column chromatography (n-hexane / ethyl acetate, 7:2) to obtain 7.7 g of solid, namely 3-Methyl-1,2,5-oxadiazole-2-oxide-4-carboxylic acid (3,5,6-trimethylpyrazin-2-yl)methanol ester (HK-003). HPLC: 99.16%, ESI-MS: 277.1 (M-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com