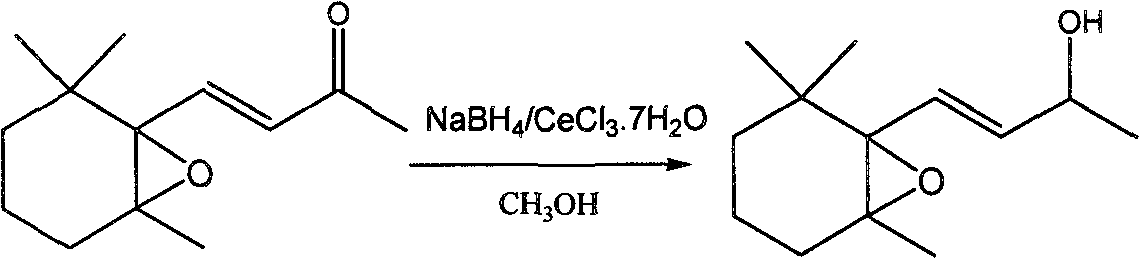Preparation method of 5,6-epoxy-5,6-dihydro-beta-ionol
A technology of ionol and ionone, applied in 5 fields, can solve problems such as compound instability and easy polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] At room temperature, 5,6-epoxy-5,6-dihydro-β-ionone 0.563g (97.10%, 2.63mmol) was dissolved in 9.21ml of anhydrous methanol, and 0.984g of cerium chloride heptahydrate was added ( 2.63mmol) was stirred until dissolved, then slowly added 0.10g (2.63mmol) of sodium borohydride within 5 minutes, continued to stir for 5 minutes after the addition was complete, and ended the reaction, and adjusted the pH to neutral with 5% dilute hydrochloric acid. Extract with ether, filter with a glass funnel, combine the filtrates, anhydrous MgSO 4 Let dry overnight. After filtration, the solvent was distilled off to obtain 0.54 g of the product with a GC content of 93.86% and a yield of 91.84%. After separation by silica gel column, the mixed solvent of petroleum ether and ethyl acetate was used as eluent, the volume ratio was 10:1, and the solvent was evaporated to obtain colorless 5,6-epoxy-5,6-dihydro-β- Ionol, the purity is greater than 98.00%.
[0021] Infrared spectral data:
...
Embodiment 2
[0029] Under ice-water bath, dissolve 0.835g (97.10%, 3.90mmol) of 5,6-epoxy-5,6-dihydro-β-ionone in 5.85ml of anhydrous methanol, and add 0.967g of anhydrous cerium chloride (3.90mmol) was stirred until dissolved, then slowly added 0.148g (3.90mmol) of sodium borohydride within 5 minutes, continued to stir for 5 minutes, ended the reaction, and adjusted the pH to neutral with 5% dilute hydrochloric acid by mass fraction. Extract with ether, filter with a glass funnel, combine the filtrates, anhydrous MgSO 4 Let dry overnight. The next day, it was filtered and the solvent was distilled off to obtain 0.78 g of the product with a GC content of 92.15% and a yield of 87.80%. After separation by silica gel column, the mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 10:1 was used as eluent to rinse, and the colorless 5,6-epoxy-5,6-dihydro- β-ionol, the purity is 98.00%.
Embodiment 3
[0031] _____Under an ice-water bath, dissolve 1.092g (97.10%, 5.10mmol) of 5,6-epoxy-5,6-dihydro-β-ionone in 22.95ml of anhydrous methanol, and add anhydrous cerium chloride After stirring 1.265g (5.10mmol) until dissolved, slowly add 0.194g (5.10mmol) of sodium borohydride within 5 minutes, continue stirring for 5 minutes to end the reaction, and adjust the pH to neutral with 5% dilute hydrochloric acid. Extract with ether, filter with a glass funnel, and use anhydrous MgSO 4 Let dry overnight. The next day, it was filtered and the solvent was distilled off to obtain 1.04 g of the product with a GC content of 93.18% and a yield of 90.53%. Separation on a silica gel column, eluting with a mixed solvent of petroleum ether and ethyl acetate as eluent, the volume ratio is 10:1, and the colorless 5,6-epoxy-5,6-dihydro- β-ionol, 97.95% pure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com