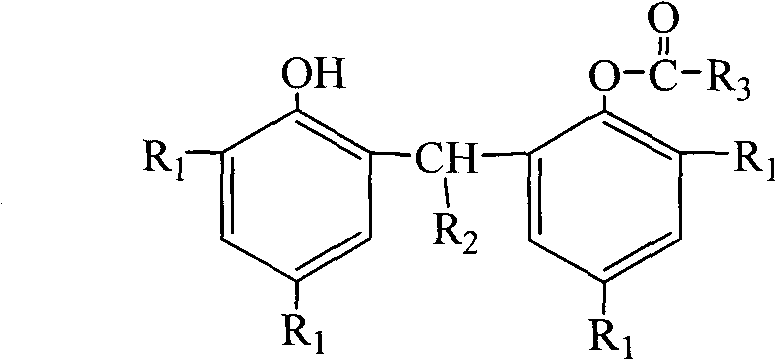
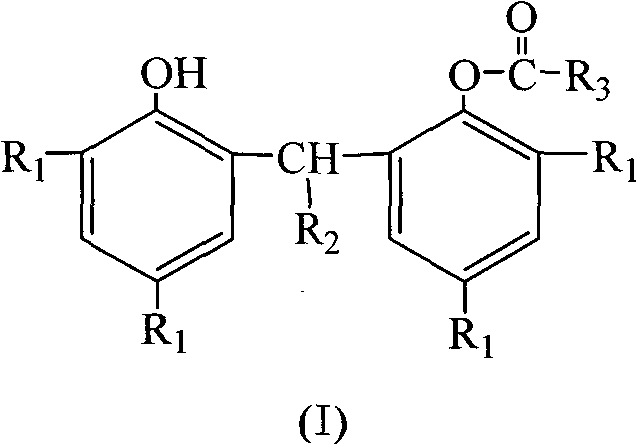
Preparation method of bisphenol monocarboxylic ester compound antioxidant
A bisphenol monocarboxylate and phenolic compound technology, which is applied in the field of preparation of bisphenol monocarboxylate compound antioxidants, can solve unfavorable large-scale industrial production, low recovery value of triethylamine, and partial color of product appearance Huang and other problems, to achieve the effect of facilitating industrial production, high product yield, and inhibiting polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In a 5000mL five-necked flask equipped with a stirrer, thermometer, acryloyl chloride addition funnel, triethylamine addition funnel, and reflux cooler, add 2,2'-methylenebis(4-methyl-6-tert-butylphenol) ) 340.50g (1.00mol), 1800mL of n-heptane. Replace the air in the kettle with nitrogen, heat and stir, and raise the temperature to 57-60°C. Keep constant at this temperature, start dripping 99.56g (1.10mol) of acryloyl chloride, and finish adding in 40-50 minutes. While acryloyl chloride was dripped, 121.43g (1.20mol) of triethylamine raw material was dripped, and the time for dripping the triethylamine raw material was 70-80 minutes. After adding the triethylamine raw material, the reaction solution is heated to 63-65°C. At this temperature, the reaction is kept for 120 to 130 minutes.
[0037] After the completion of the reaction, the resulting reaction mixture is a solid-liquid mixture. After filtration, the filter cake triethylamine hydrochloride was washed with 20...
Embodiment 2
[0040] In a 5000mL five-necked flask equipped with a stirrer, thermometer, acryloyl chloride addition funnel, triethylamine addition funnel, and reflux cooler, add 2,2'-methylenebis(4-methyl-6-tert-butylphenol) ) 340.50g (1.00mol), 1800mL petroleum ether. Replace the air in the kettle with nitrogen, heat and stir, and raise the temperature to 63-65°C. Keep constant at this temperature, start dripping 108.61g (1.20mol) raw material of acryloyl chloride, and finish adding in 40-50 minutes. While acryloyl chloride was dripped, 131.55g (1.30mol) of triethylamine raw material was dripped, and the time for dripping the triethylamine raw material was 70-80 minutes. After adding the triethylamine raw material, the reaction solution is heated to 67-70°C. At this temperature, the reaction is kept for 150-160 minutes.
[0041] After the completion of the reaction, the resulting reaction mixture is a solid-liquid mixture. After filtering, the filter cake triethylamine hydrochloride was w...
Embodiment 3
[0044] In a 5000mL five-necked flask equipped with a stirrer, thermometer, acryloyl chloride addition funnel, triethylamine addition funnel, and reflux cooler, add 2,2'-methylenebis(4-methyl-6-tert-butylphenol) ) 340.50g (1.00mol), 1800mL petroleum ether. Replace the air in the kettle with nitrogen, heat and stir, and raise the temperature to 64-66°C. Keep constant at this temperature, start dripping 117.66g (1.30mol) of acryloyl chloride, and finish adding in 40-50 minutes. While acryloyl chloride was dripped, 141.67g (1.40mol) of triethylamine was dripped, and the time for dripping the triethylamine raw material was 70-80 minutes. After adding the triethylamine raw material, the reaction solution is heated to 72-75°C. At this temperature, the reaction is kept for 120 to 130 minutes.
[0045] After the completion of the reaction, the resulting reaction mixture is a solid-liquid mixture. After filtering, the filter cake triethylamine hydrochloride was washed with 200mL petrol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com