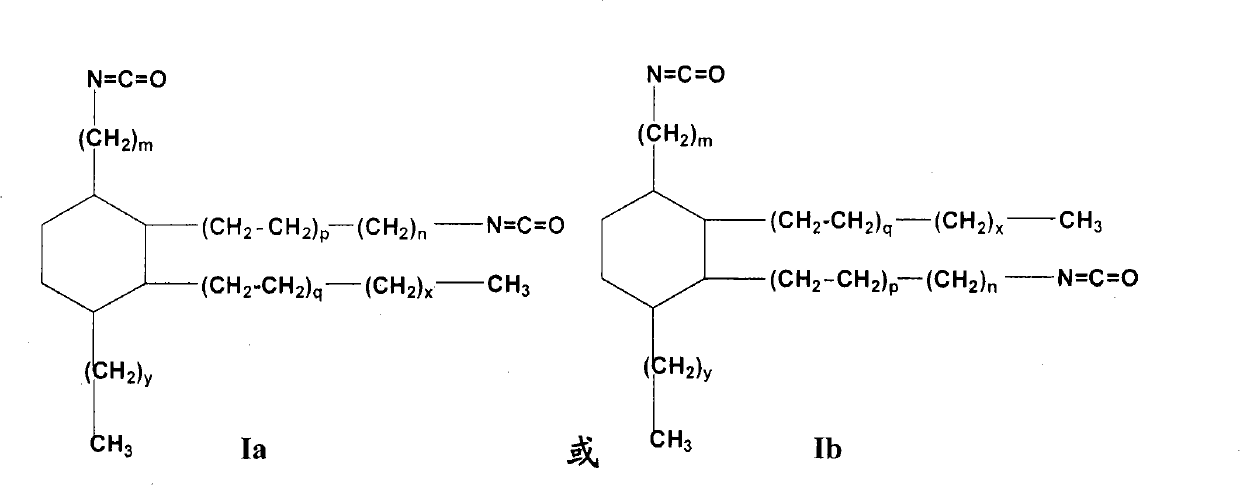
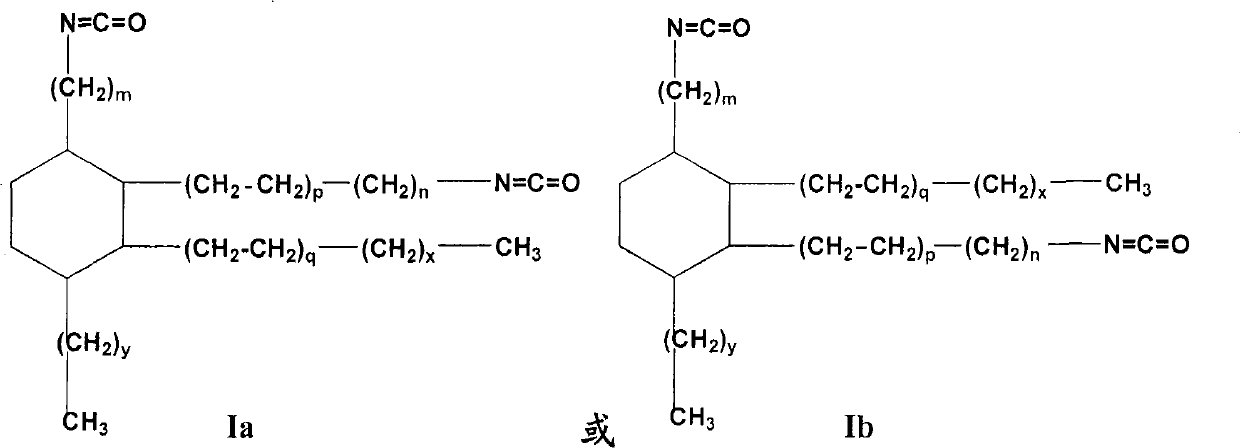
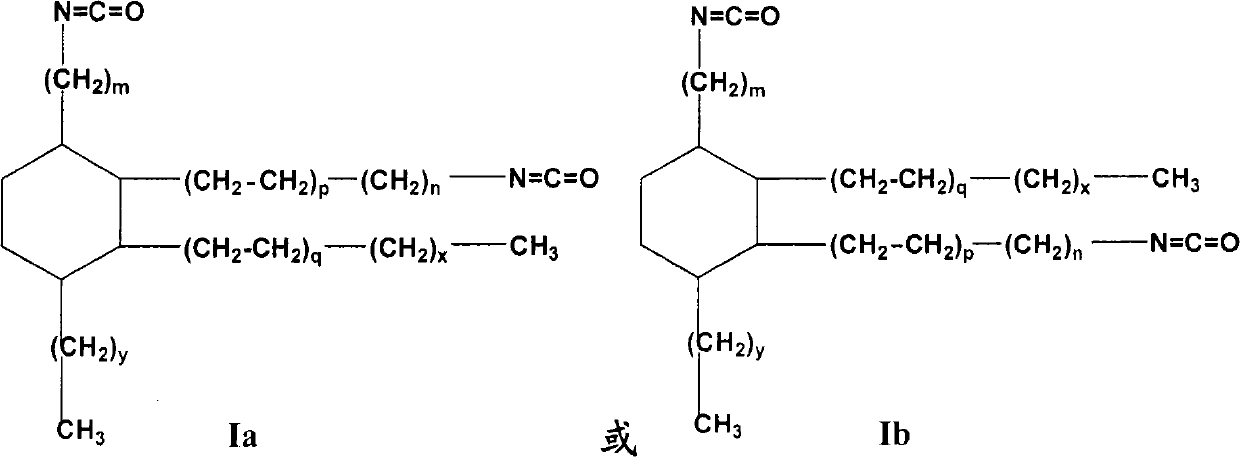
Isocyanate alkyl substituted cyclohexane and preparation method as well as application thereof
A technology of isocyanate and cyclohexane, which is applied in the field of cyclohexane preparation, can solve the problems of harsh process conditions, difficult industrialization, high toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0129] In one embodiment of the preparation method of the second aspect of the present invention, the following steps are adopted: add xylene in the hydrogenation autoclave, then add Pd (5%) / activated carbon catalyst, add dimer acid, and the amount of dimer acid in the mixture The concentration is 10-50% based on the total amount of solvent, and the weight ratio of dimer acid to palladium is 99:1, heated to 150-200°C, reacted at 1.5-2.0MPa for 6 hours, after the hydrogenation absorption is completed, cool down and stop Stir, filter the catalyst, apply mechanically, and dry the reaction mixture.
[0130] Add more than 1.05mol of acyl chloride agent to the above dried reaction mixture, react at 20-70°C for 6-8 hours, and distill off unreacted acyl chloride agent.
[0131] Add 2.2 moles of sodium azide to the above reaction mixture from which the acyl chlorination agent has been removed, react at 80° C. for 16 hours, and filter out the generated sodium chloride.
[0132] The rea...
specific Embodiment approach
[0137] The present invention is further illustrated below through specific intermediates and examples, but it should be understood that these intermediates and examples are only used for more detailed description, and should not be interpreted as being used to limit the present invention in any form. invention.
[0138] The present invention provides general and / or specific descriptions of the materials and test methods used in the tests. While many of the materials and methods of manipulation which are employed for the purposes of the invention are well known in the art, the invention has been described here in as much detail as possible. It will be clear to those skilled in the art that in the following, unless otherwise specified, the materials and operation methods used in the present invention are well known in the art. In particular, the dimer acid of the starting material formula Ia-1 or Ib-1 used in the present invention is prepared by Diels-Alder reaction with octade...
Embodiment 1
[0139] Embodiment 1,1-pentyl-2-heptyl-3, the preparation of 4-two (8-isocyanate octyl)-cyclohexane
[0140] Raw materials: dimer acid is a dimer acid of the following structure:
[0141]
[0142] step 1: Put 350g xylene and 30g Pd (5%) / activated carbon catalyst (wet: dry weight 15g, 0.45g Pd) suspension into 2 liters of hydrogenation autoclave, then add 320g dimer acid (98%), draw separately Vacuum, nitrogen replacement, hydrogen replacement. The reaction mixture was stirred at 170-190° C., 1.5-2.0 Mp for about 7.5 hours, until the hydrogen absorption was complete, and the stirring was stopped. The material was filtered off under nitrogen pressure, leaving the catalyst in the filter. Use 350g xylene to backwash the catalyst into the reactor, add 320g dimer acid, repeat the hydrogenation, the catalyst activity does not decrease, carry out the hydrogenation 5 times in the same way, and reuse the catalyst 4 times, the obtained reaction mixture Heated and dehydrated to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com