Bergenin and cetirizine dihydrochloride compound oral administration preparation
A technology of cetirizine hydrochloride and petrolatum, which is applied in the field of medicine, can solve the problems of high medication frequency, low bioavailability of petrolatin, can not meet the long-term medication requirements of patients with chronic bronchitis and bronchial asthma, etc. Significant, long-lasting, well-tolerated effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
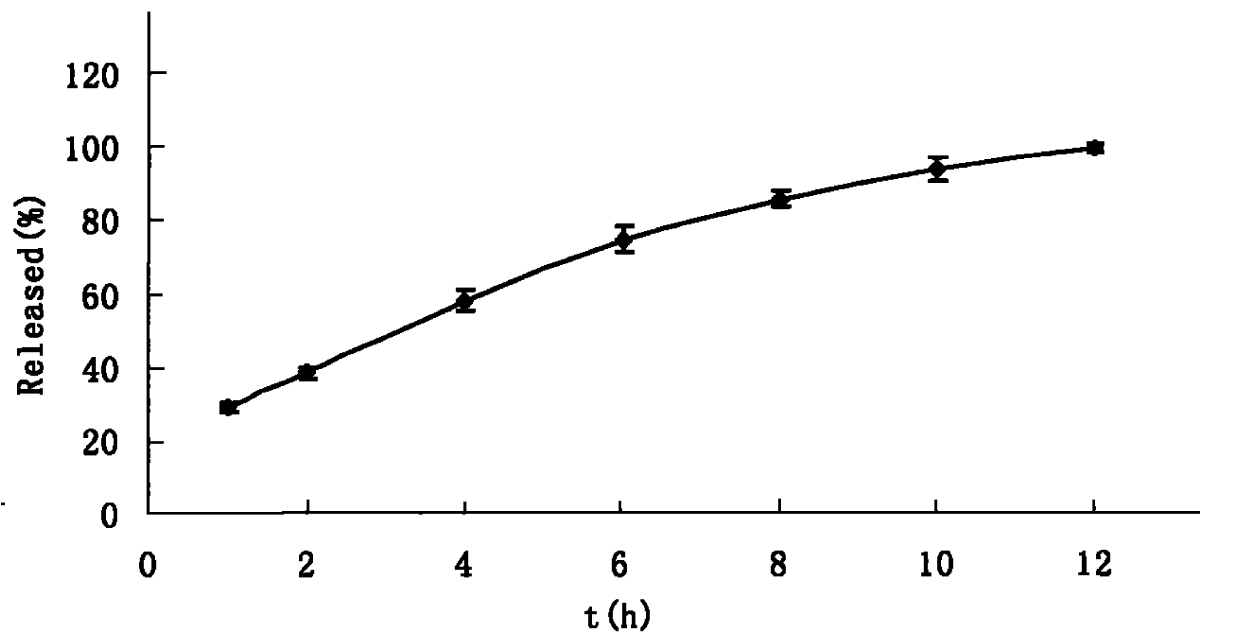
Embodiment 1
[0106] Sustained release tablet core:
[0107] Begenin 187.5g
[0108] Hydroxypropyl Methyl Cellulose K4M 27g
[0109] Lactose 39g
[0110] Sodium bicarbonate 45g
[0111] 5% PVPk30 ethanol solution appropriate amount
[0112] Magnesium Stearate 1.5g
[0113] Made into 1000 pieces
[0114] Immediate Release Coating:
[0115] Cetirizine Hydrochloride 5g
[0116] Easy Release 4g
[0117] Made into 1000 pieces
[0118] Pass the raw and auxiliary materials through 80 or 100 mesh sieves respectively, mix petgenin, lactose, and HPMC according to the equal amount incremental method, use 5% PVPk30 ethanol solution as the binder to make soft materials, and pass through 20-24 mesh sieves to granulate , dried at 45-60°C for 1.5-2 hours, and granulated for later use. Sodium bicarbonate is made into a soft material with 5% PVPk30 ethanol solution as a binder, dried at 40-50°C for 1.5-2 hours, and granulated for later use. The two kinds of granules are uniformly mixed according t...
Embodiment 2
[0141] Sustained release tablet core:
[0142] Begenin 375g
[0143] Hydroxypropyl Methyl Cellulose K4M 40g
[0144] Hydroxypropyl Methyl Cellulose E5 020g
[0145] Lactose 75g
[0146] Sodium bicarbonate 90g
[0147] Proper amount of PVPk30 ethanol solution
[0149] Made into 1000 pieces
[0150] Immediate Release Coating:
[0151] Cetirizine Hydrochloride 10g
[0152] Opadry 9g
[0153] Made into 1000 pieces
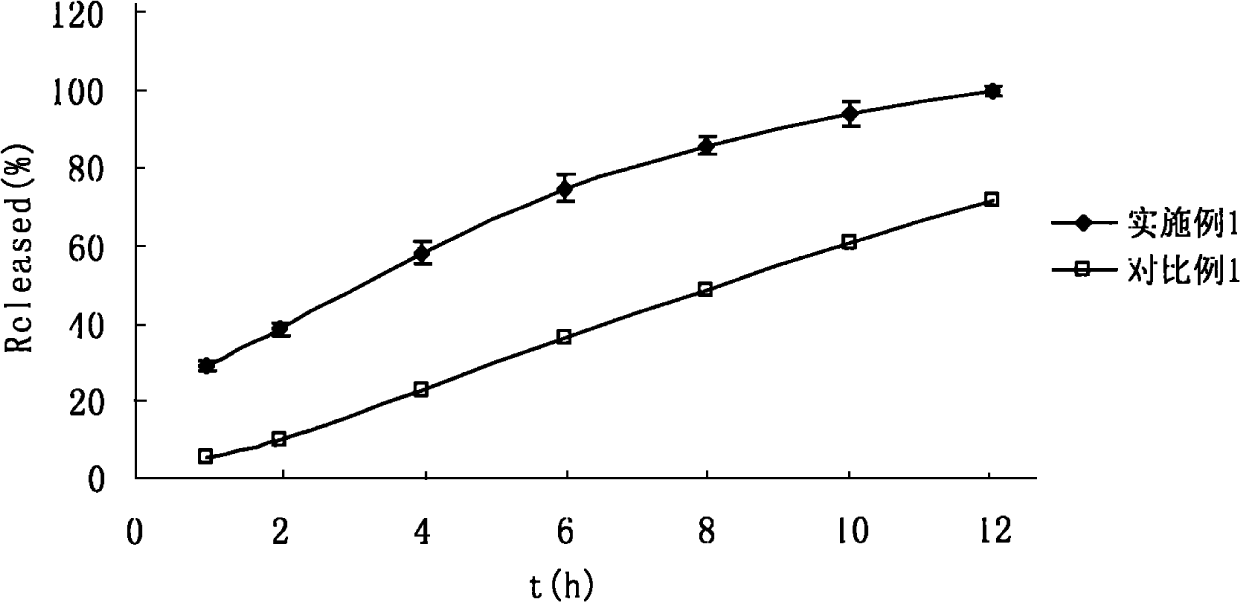
[0154] Separately pass lactose, HPMC and petogenin through 80 or 100 mesh sieves, mix the above-mentioned raw and auxiliary materials evenly according to the method of equal addition, use 5-10% PVPk30 ethanol solution as a binder to make soft materials, and pass through 20-24 mesh sieves Granulate, dry at 45-60°C for 2-3 hours, and prepare the granules for later use. Sodium bicarbonate is made into a soft material with 5-10% PVPk30 ethanol solution as a binder, dried at 40-50°C for 1.5-2 hours, and granulated for later use. Th...
Embodiment 3
[0156] Sustained release tablet core:
[0157] Begenin 125g
[0158] Hydroxypropyl Methyl Cellulose K4M 20g
[0159] Mannitol 30g
[0160] Sodium carbonate 25g
[0161] Proper amount of PVPk30 ethanol solution
[0163] Made into 1000 pieces
[0164] Immediate Release Coating:
[0165] Dithirizine Hydrochloride 2g
[0166] Easy Release 4g
[0167] Made into 1000 pieces
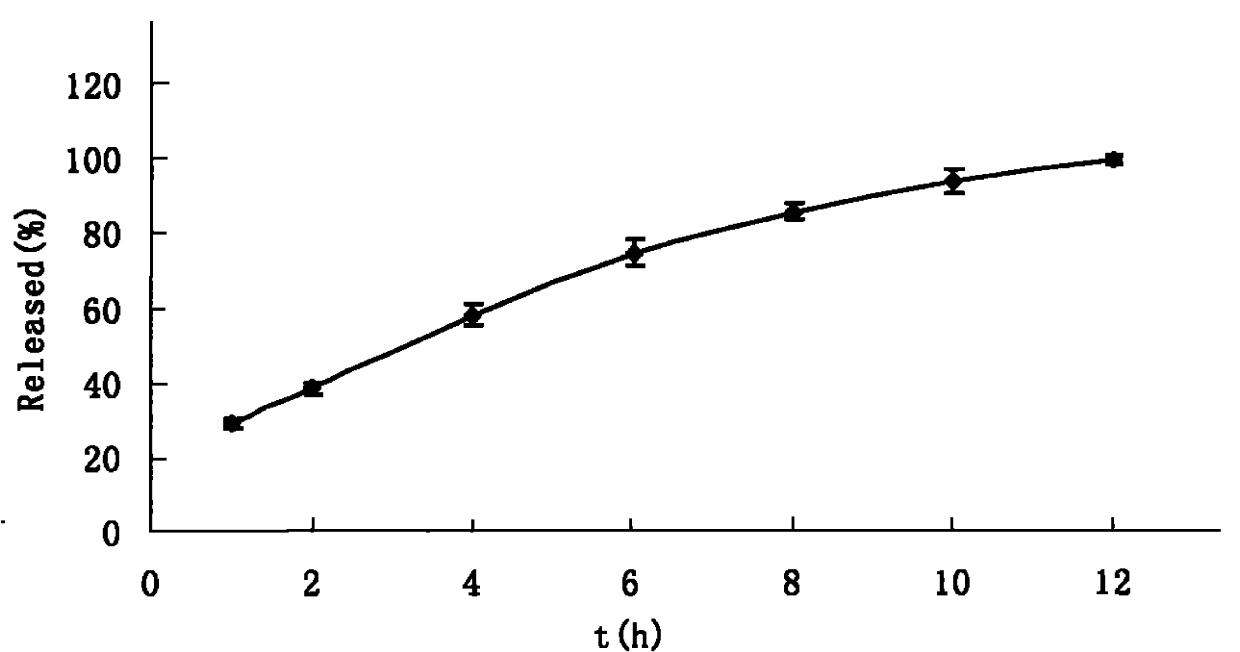
[0168] Sieve mannitol, HPMC K4M, and petracenin through 80 or 100 mesh sieves, mix the above-mentioned raw and auxiliary materials evenly according to the method of equal volume addition, use 5-10% PVPk30 ethanol solution as a binder to make a soft material, and pass 20-24 Granulate with a mesh sieve, dry at 45-60°C for 1-2 hours, and prepare the granules for later use. Sodium carbonate is made into a soft material with 5-10% PVPk30 ethanol solution as a binder, dried at 40-50°C for 1.5-2 hours, and granulated for later use. The two kinds of granules are mixed uniformly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com