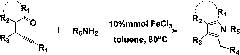Method for preparing iron-catalyzed pyrrole and pyrrole cyclic compounds
A compound, pyrrole technology, which is applied in the field of preparation of pyrrole and pyrrolocyclic compounds, and achieves the effects of low price, green catalytic system, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1) Preparation of 4-pentynone compound 1a
[0025]
[0026] Into a 50 mL round bottom flask equipped with a magnetic stirrer was added anhydrous N,N-dimethylformamide (DMF) (15 mL), N-acetoacetanilide (1.77 g, 10 mmol) and anhydrous potassium carbonate (1.66 g , 12mmol), slowly add 3-bromopropyne (1.43g, 12mmol) dropwise at a room temperature of 20°C under stirring, at a rate of 1 drop / 2 seconds. Stirring was continued for 12 hours to obtain a light yellow reaction liquid. The reaction solution was poured into saturated aqueous sodium chloride solution (20mL), extracted with dichloromethane (3×15mL), and the organic phases were combined, then backwashed with water (3×10mL), dried over anhydrous calcium chloride, Steps such as suction filter, vacuum distillation obtain viscous solid, through silica gel column chromatography (eluent is V 石油醚 :V 乙酸乙酯 =10:3) to obtain 1.25 g of white solid, the structure of the product was confirmed by NMR and MS to be 4-pentynone com...
Embodiment 2
[0033] Replace 4-chloroaniline in "Example 1" with 2-naphthylamine, use benzene as solvent, and the reaction temperature is 50°C. Other conditions are the same as "Example 1". The experimental results are shown in Table 1.
[0034]
[0035] Spectrum analysis data 2b:
[0036] 1 H NMR (500MHz, CDCl 3 )δ=2.04(s, 3H), 2.39(s, 3H), 6.24(s, 1H), 7.07-7.97(m, 13H); 13 CNMR (125MHz, CDCl 3 )δ=12.13, 12.55, 104.10, 114.14, 119.52, 123.21, 125.52, 126.65, 126.74, 127.59, 127.74, 128.64, 128.89, 129.17, 132.51, 133.00, 134.73, 1368.028 -1 ) 3257, 3057, 1638, 1596, 1577, 1498, 1309, 1257, 795, 759, 690;
Embodiment 3
[0038] Replace 4-chloroaniline in "Example 1" with benzylamine, meanwhile, use FeBr 3 , other conditions are the same as "Example 1", and the experimental results are shown in Table 1.
[0039]
[0040] Spectral analysis data 2c:
[0041] 1 H NMR (500MHz, CDCl 3 )δ=2.15(s, 3H), 2.52(s, 3H), 5.05(s, 2H), 6.16(s, 1H), 6.90(d, J=8.0Hz, 2H), 7.06-7.34(m, 5H ), 7.49(s, 1H), 7.59(d, J=8.0Hz, 2H); 13 C NMR (125MHz, CDCl 3 )δ=11.17, 12.21, 46.68, 61.49, 104.32, 113.96, 119.72, 123.39, 125.45, 127.36, 128.20, 128.84, 128.85, 134.54, 136.90, 138.63, 164.09; -1 )3249, 3027, 1638, 1536, 1495, 1435, 1250, 753, 691; MS m / z Calcd: 304.2; Found: 305.2 [(M+1) + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com