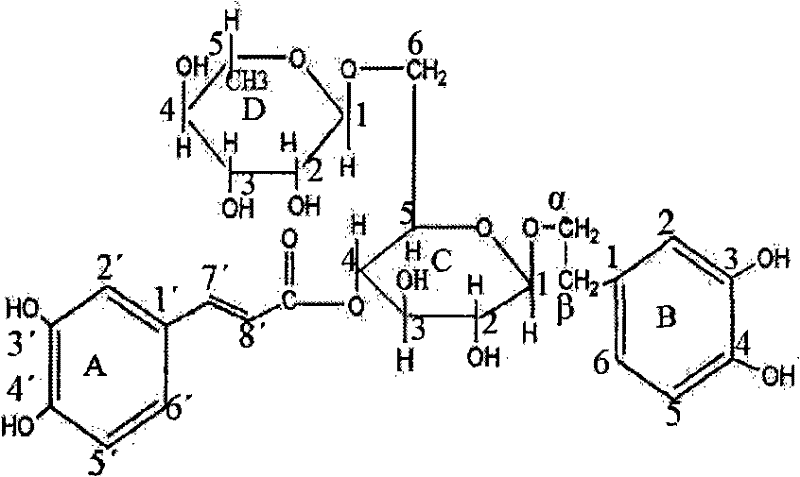
Application of forsythiaside A in preparation of drugs for preventing and treating cardiovascular and cerebrovascular diseases
A technology of forsythiaside and medicine is applied in the application field of forsythiaside A in the preparation of medicines for preventing and treating cardiovascular and cerebrovascular diseases, which can solve the problems of never-before-seen medicines for cardiovascular and cerebrovascular diseases, and achieve the effect of strong pharmacological action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
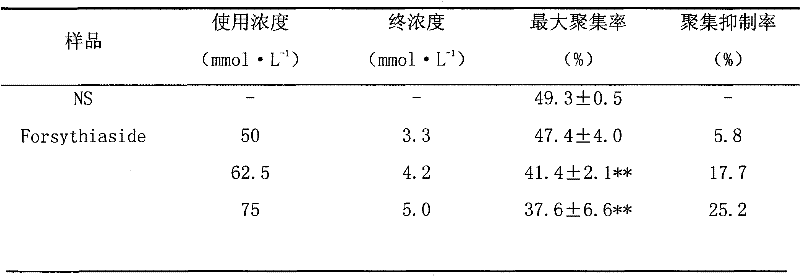
[0013] Example 1 Effect of forsythiaside A on ADP-induced platelet aggregation in rats in vitro
[0014] Blood was collected from the abdominal aorta of rats (Wistar, clean level II), anticoagulated with 3.8% sodium citrate 1:9, 500r·min -1 Centrifuge for 5 minutes to take the supernatant to obtain platelet-rich plasma (PRP). The remaining part 2500r·min -1 After centrifugation for 10 min, the supernatant was taken to obtain platelet-poor plasma (PPP). Dilute PRP with PPP to bring platelet count to 3×10 11 / L.
[0015] Take 265 μL PRP and place it in a turbidimetric tube, add forsythiaside (content greater than 95%) solution (final concentration is 2.1, 2.6, 3.1, 3.6, 4.1, 6.2, 8.2mmol·L -1 ) 20 μL, add an equal volume of normal saline to the blank control tube, pre-warm at 37°C for 3 minutes, start the platelet aggregation coagulation factor analyzer, and quickly add 2mmol·L -1 ADP15μL, record the platelet aggregation curve, and calculate the inhibition rate of platelet...
Embodiment 2
[0022] Example 2 Effect of Forsythiaside A on Rat Plasma Prothrombin Time (PT)
[0023] Rat abdominal aorta blood collection, 3.8% sodium citrate 1:9 anticoagulant, 2500r·min -1 After centrifugation for 10 min, the supernatant was taken to obtain PPP. Pre-warm the PT reagent in the reagent pre-warming well at 37°C for more than 10 minutes, and shake well before use. Take 50 μL of PPP and place it in a turbidimetric tube, add forsythiaside A solution (final concentration is 8.3, 12.5, 16.6 mmol L -1 ) 30 μL, add an equal volume of normal saline to the control tube, pre-warm at 37 °C for 180 seconds, add 100 μL of PT reagent at 37 °C, start the instrument immediately for automatic testing, and record the results.
[0024] The results showed that: Forsythiaside can significantly prolong the isolated plasma PT of rats, and the prolonging effect is more obvious with the increase of the dose (Table 2). The final concentration is 16.6mmol·L -1 Forsythiaside prolongs PT for more t...
Embodiment 3
[0028] Example 3 Effect of Forsythiaside A on Rat Plasma Thrombin Time (TT)
[0029] The PPP preparation method is the same as above, take the TT reagent out of the refrigerator and let it stand at room temperature for 10 minutes, and the temperature is equilibrated to room temperature. Take 80 μL of PPP and place it in a turbidimetric tube, add forsythiaside solution (final concentration is 7.1, 10.7, 14.2 mmol L -1 ) 30 μL, add an equal volume of normal saline to the control tube, pre-warm at 37°C for 180 seconds, add 100 μL of TT reagent, immediately start the instrument for automatic testing, and record the results.
[0030] The results showed that: forsythiaside A can significantly prolong the isolated plasma TT in rats, and there is an obvious dose-effect relationship (Table 3).
[0031] The influence of table 3 forsythiaside A on rat isolated plasma TT ( n=6)
[0032]
[0033] *P<0.05 vs NS, ***P<0.001 vs NS
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com