Active fragment of anti-opioid peptide
An active fragment, anti-opioid peptide technology, applied in the direction of DNA/RNA fragments, peptides, peptide sources, etc., can solve the problems of limited efficacy of acupuncture analgesia and tolerance problems of acupuncture, so as to be easily degraded and reduced. Morphine dosage, the effect of facilitating development and research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1. Obtainment and functional verification of anti-opioid peptide 21Kd and its active fragments
[0070] 1. Acquisition of pig brain anti-opioid peptide 21Kd
[0071] 1. Discovery of anti-opioid peptides
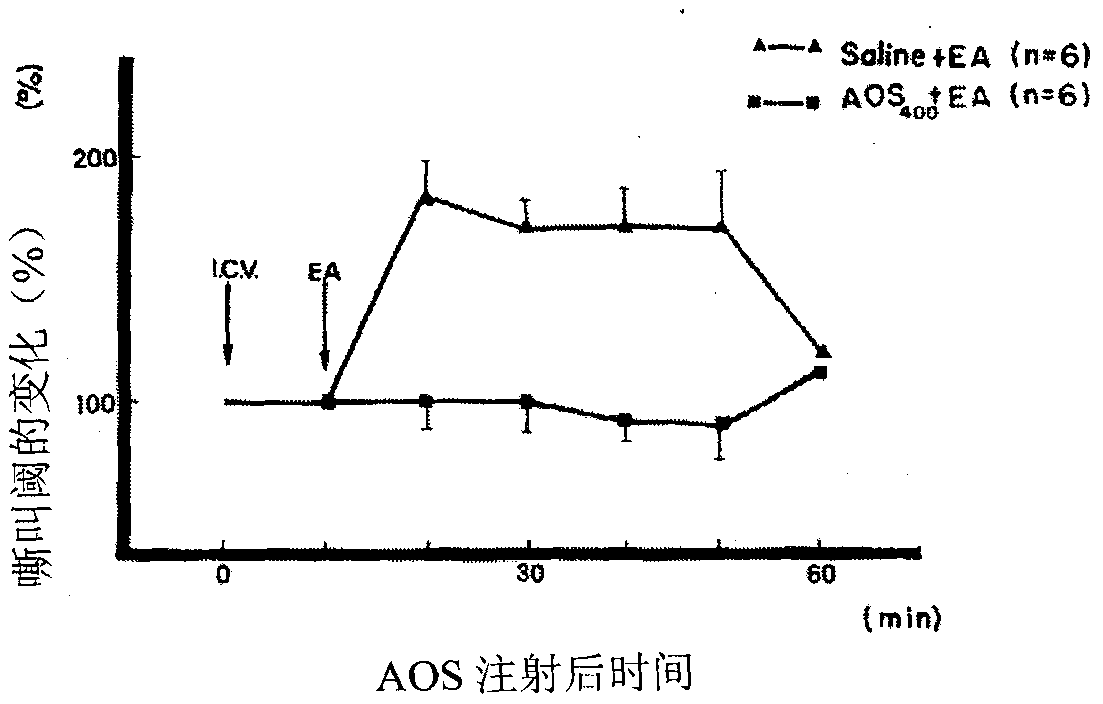
[0072] In the isolated mouse vas deferens specimens, stimulation with a square wave pulse (0.1MS width, 1 time / second) can cause contraction (see the literature for the specific method: J. Hughes et al (1975) Effect of morphine on adrenergic transmission in the Mouse Vas deferens.Assessment of agonist and antagonistpotencies of Narcotic analgesics 53,371-381), then, successively add morphine hydrochloride (3.2nM) or dog brain extract (AOS, 200μg / mL) (for dog brain extract The concentrated solution was successively filtered through Sephadex G-50 gel (purchased from pharmacia company), and after carboxymethyl-cellulose (CM-C, purchased from H.Reeve-Angel&.Co.Ltd company) cation exchange chromatography, the sample was obtained ), to observe the effect on the con...
Embodiment 2
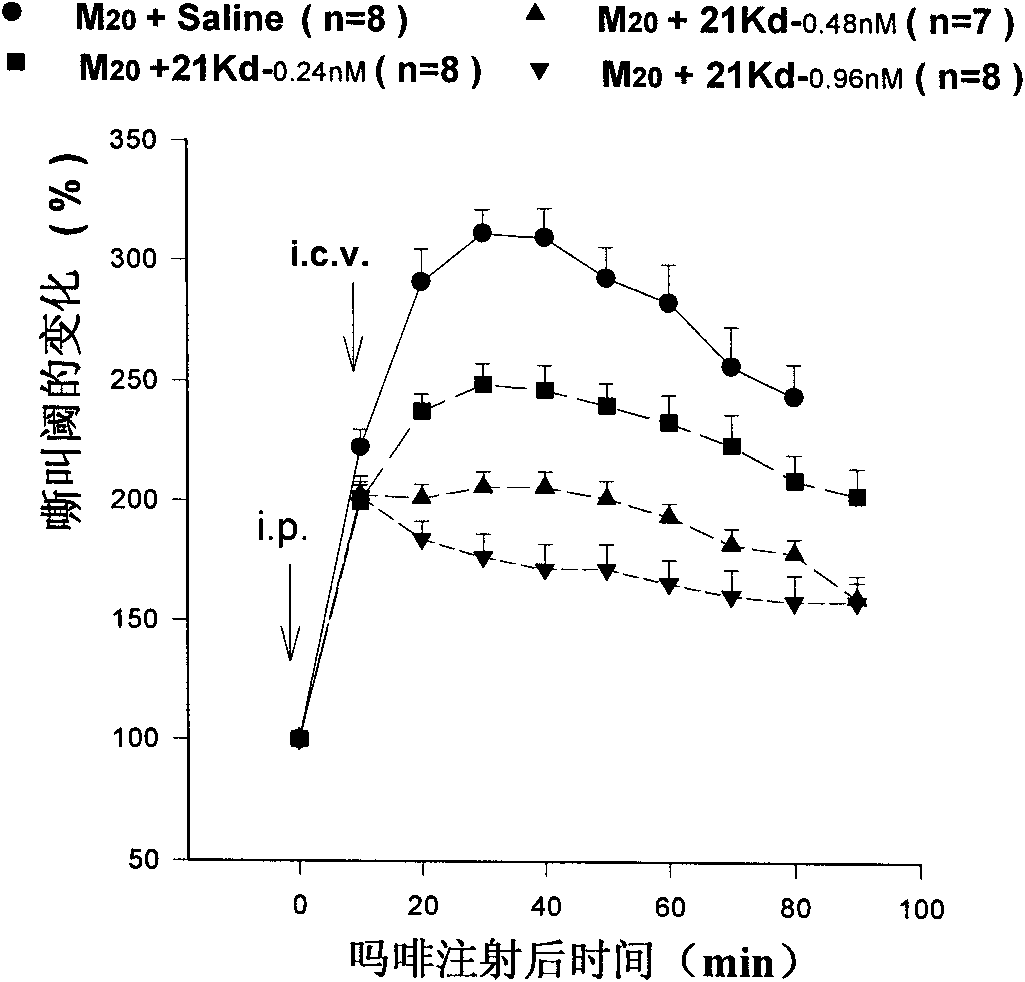
[0087] Example 2, Detection of the relationship between anti-opioid peptide 21Kd and morphine tolerance and dependence
[0088] 1. Obtainment and functional detection of soluble single-chain variable region antibody fragment against opioid peptide 21Kd
[0089] Using phage display technology, using the human prophage antibody library constructed by Daniele Sblattero and Andrew Bradbury of the International School for Advanced Studies (SISSA) in Italy [For the construction method, see: Sblattero D, Lou J L, Marzari R, et al.In vivo recombination as atool to generate molecular diversity in phage antibody libraries. Reviews in Molecular Biotechnology. 2001, 74: 303-315], a soluble single-chain variable region antibody fragment with a molecular weight of about 20,000 Daltons of anti-opioid peptide 21Kd was screened out from the phage antibody library, Abbreviated as ScFv. Intraperitoneal injection of morphine (M 10, 10 mg / kg) 10 minutes later, the ScFv was injected into the late...
Embodiment 3
[0096] Example 3. Detection of the role of NMDA receptors and NOS in antagonizing the antinociceptive effect of morphine on the anti-opioid peptide 21Kd and its active fragments
[0097] Studies have shown that excitatory amino acid NMDA receptors and nitric oxide synthase system (NOS) play a crucial role in the process of morphine tolerance and dependence in adult mice, and mice were treated with NMDA receptor antagonists or NOS inhibitors After pretreatment, it can inhibit the formation of morphine tolerance and dependence, and prevent the appearance of withdrawal symptoms [Adams ML, Kalicki JM, Meyer ER, Cicero TJ (1993) Inhibition of the morphine withdrawal syndrome by nitric oxide synthase inhibitor, NG-Nitro -L-arginine methyl ester. Life Sci52: PL245-PL249; Cappendijk SL, Vries R, Dzoljic MR (1993) Inhibitory effect of nitric oxide (NO) synthase inhibitors on naloxone-precipitated withdrawal syndrome in morphine-dependent mice. Neurosci7 Lett 162: -100; Herman BH, Vocci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com