Method for synthesizing N-isopropylhydroxyla
A technology of isopropylhydroxylamine and propylhydroxylamine salt, applied in the direction of organic chemistry and the like, can solve the problems of complex process, low yield of N-isopropylhydroxylamine, etc., and achieves abundant raw material sources, mild reaction conditions and low production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
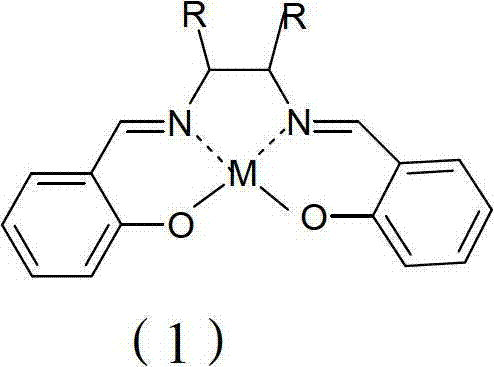
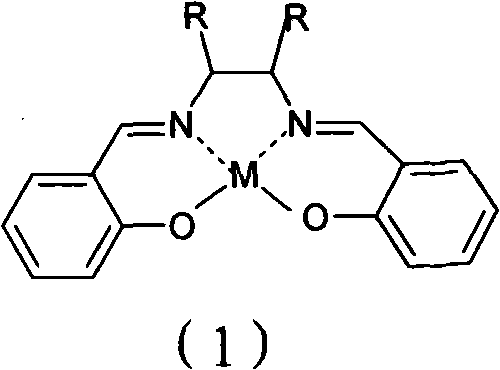
[0025] In a 1000L reactor, add 200Kg of diisopropylamine and 200g of Mn (Salen) catalyst, raise the temperature to 60°C, slowly add 500Kg of 30% aqueous hydrogen peroxide solution dropwise, and complete the dropwise addition in 3 hours. Keep warm for 1h. Then add 214L mass percent concentration and be 37% hydrochloric acid solution, hydrolyze under acidic condition, concentrate, cooling crystallization makes 201Kg isopropyl hydroxylamine hydrochloride; Add 600L methyl tert-butyl Ether and 170Kg concentration are 50% sodium hydroxide aqueous solution, separate and obtain 119Kg of 99% isopropyl hydroxylamine, and yield is 88% (calculated as diisopropylamine).
Embodiment 2
[0027] In a 1000L reactor, add 200Kg of diisopropylamine and 300g of Mn(Salen-Ph) catalyst, raise the temperature to 60°C, slowly add 600Kg of 25% aqueous hydrogen peroxide solution dropwise, dropwise after 4 hours, dropwise Complete and continue to insulate for 1 hour, then add 230L of hydrochloric acid solution with a mass percentage concentration of 37%, hydrolyze, concentrate, and cool to crystallize under acidic conditions to obtain 185Kg isopropyl hydroxylamine hydrochloride; add 600L of isopropyl hydroxylamine hydrochloride Tetrahydrofuran and 150Kg concentration are 50% aqueous sodium hydroxide solution, separate and obtain 89Kg of 99% isopropyl hydroxylamine, and the yield is 67% (calculated as diisopropylamine).
Embodiment 3
[0029] In a 1000L reactor, add 200Kg of diisopropylamine and 800g of Fe(Salen) catalyst, raise the temperature to 60°C, slowly add 300Kg of 50% aqueous hydrogen peroxide dropwise, and complete the dropwise addition in 2.5 hours. Insulate for 1h, then add 236L of 37% hydrochloric acid solution in mass percentage concentration, hydrolyze under acidic conditions, concentrate, cool and crystallize to obtain 195Kg isopropyl hydroxylamine hydrochloride; add 635L water and 145Kg concentration is 50% sodium hydroxide aqueous solution, separates and obtains 840Kg of 15.6% isopropyl hydroxylamine, and yield is 86% (calculated as diisopropylamine).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com