Electrochromic conducting polymer and preparation method as well as application thereof
A conductive polymer and color-induced technology, which is applied in the direction of color-changing fluorescent materials, chemical instruments and methods, electrical components, etc., to achieve the effect of good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1, preparation multi-color electrochromic conducting polymer (polyaniline doped with acid red G) of the present invention
[0036] Add 0.25g of acid red G (0.5mmol) into 25mL of hydrochloric acid solution (0.04mol / L), stir magnetically until a uniform solution is formed, then add 0.68g of ammonium persulfate (3mmol) and 0.23mL of aniline (2.5mmol) Add it into the above-mentioned hydrochloric acid solution containing acid red G, stir magnetically at 0-10°C for 24 hours, stop the reaction, filter with suction, wash the filter cake with water, ethanol and ether for several times until the washing liquid is almost colorless, and finally The product was vacuum-dried at 60°C for 24 hours to obtain a purple-black solid powder. In this embodiment, the molar ratio of aniline: ammonium persulfate: acid red G: is 1:1.2:0.2.
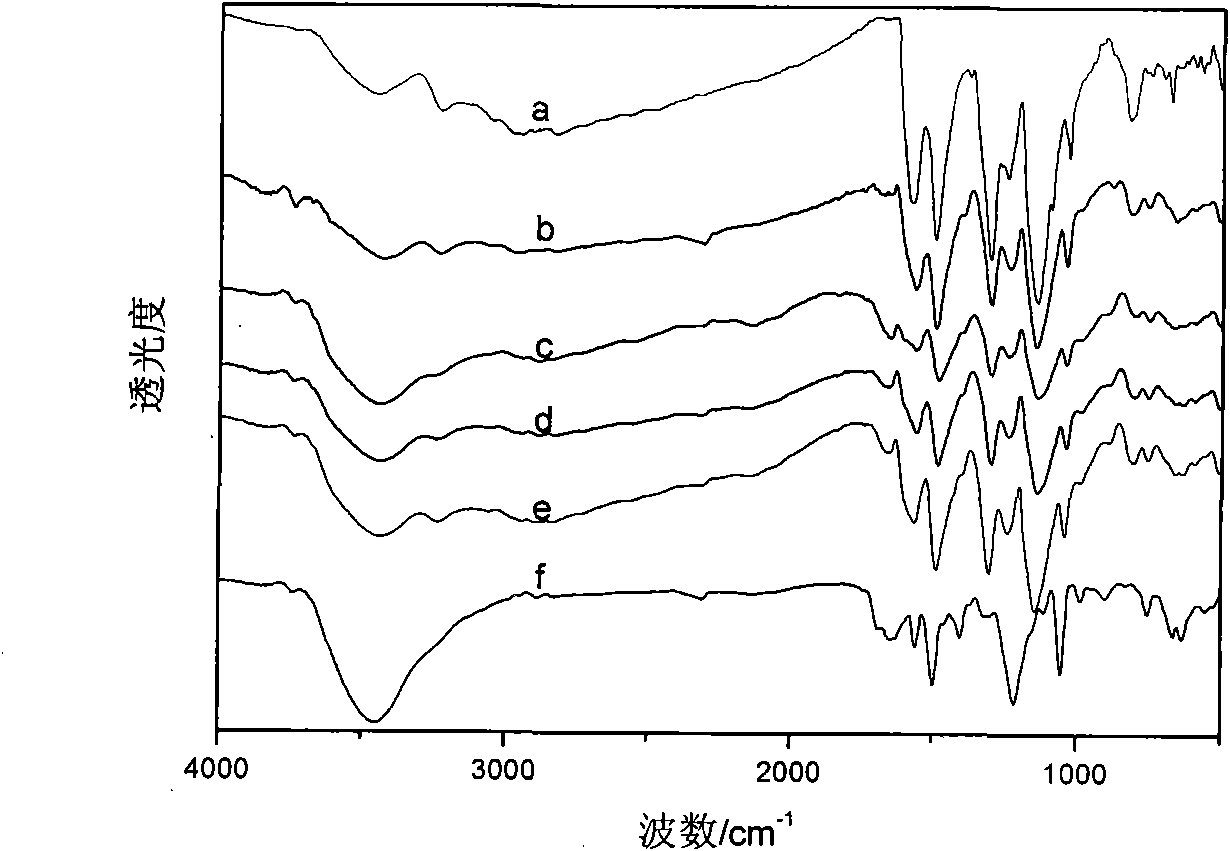
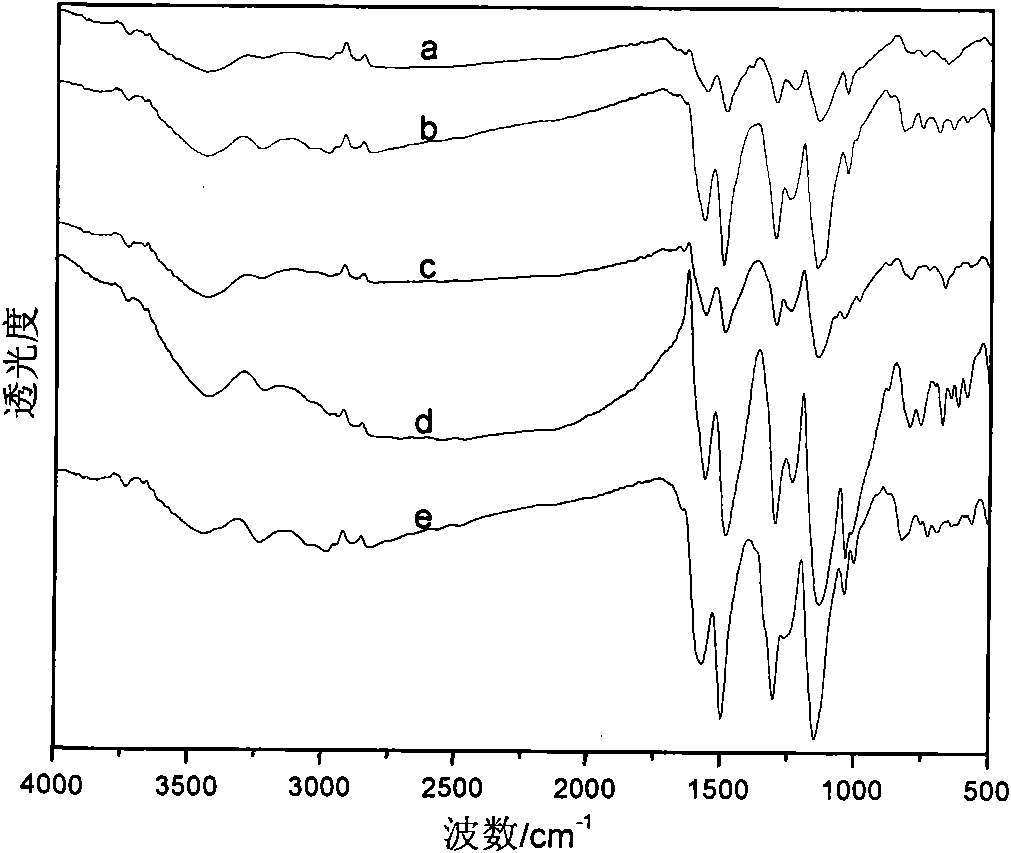
[0037] This solid powder product proves that it has the typical structure of PANI through infrared spectrum, and its infrared spectrum is as follow...
Embodiment 2
[0038] Embodiment 2, prepare the polyaniline doped with acid red G of the present invention
[0039] Add 0.51g of acid red G (1mmol) to 25mL of hydrochloric acid solution (0.08mol / L), stir magnetically until a uniform solution is formed, then add 0.68g of ammonium persulfate (3mmol) and 0.23mL of aniline (2.5mmol) into the above-mentioned hydrochloric acid solution containing acid red G, stirred magnetically at 0-10°C for 24 hours, stopped the reaction, filtered with suction, and washed the filter cake with water, ethanol and ether for several times until the washing liquid was almost colorless, and finally the product Vacuum-dried at 60°C for 24 hours to obtain a purple-black solid powder. In this example, the molar ratio of aniline:ammonium persulfate:acid red G:1:1.2:0.4.
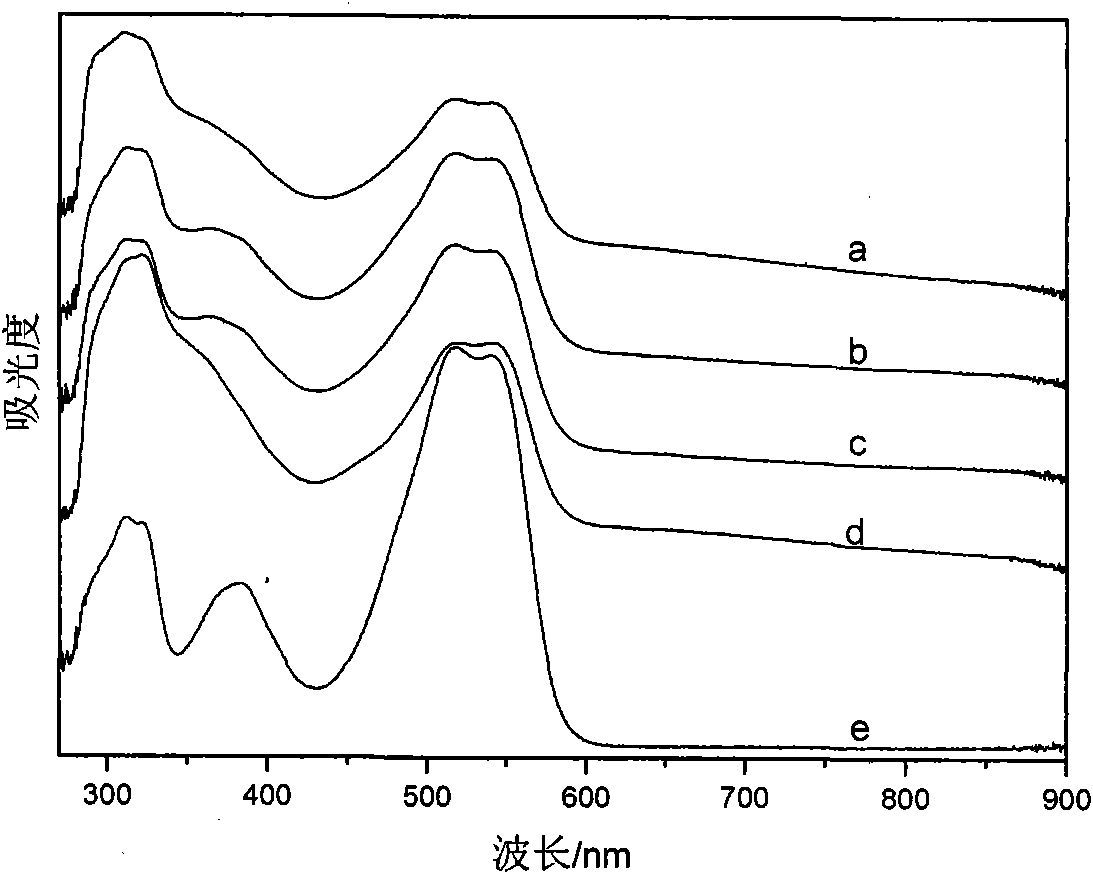
[0040] This solid powder product proves that it has the typical structure of PANI through infrared spectrum, and its infrared spectrum is as follows: figure 1 As shown in c, the ultraviolet spectrum is...
Embodiment 3
[0041] Embodiment 3, prepare the polyaniline doped with acid red G of the present invention
[0042] Add 0.76g of Acid Red G (1.5mmol) into 25mL of hydrochloric acid solution (0.12mol / L), stir magnetically until a uniform solution is formed, then add 0.68g of ammonium persulfate (3mmol) and 0.23mL of aniline (2.5mmol) Add it into the above-mentioned hydrochloric acid solution containing acid red G, stir magnetically at 0-10°C for 24 hours, stop the reaction, filter with suction, wash the filter cake with water, ethanol and ether for several times until the washing liquid is almost colorless, and finally The product was vacuum-dried at 60°C for 24 hours to obtain a purple-black solid powder. In this example, the molar ratio of aniline:ammonium persulfate:acid red G:1:1.2:0.6.
[0043] This solid powder product proves that it has the typical structure of PANI through infrared spectrum, and its infrared spectrum is as follows: figure 1 As shown in d, the UV spectrum is shown as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com