Preparation method of lithium iron phosphate
A lithium iron phosphate and ferrous phosphate technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of small specific capacity, difficult to stabilize electrical properties, poor electrical conductivity of lithium iron phosphate, etc. The effect of stable chemical properties and high electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
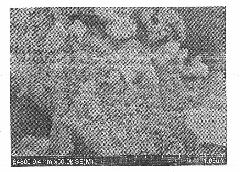
Image
Examples
Embodiment 1
[0027] Dissolve 1 mol of ferric nitrate in water to form a ferric ion solution with a concentration of 1 mol / L, add ascorbic acid that is 100% of the molar percentage of ferric ions, and then add a concentration of 1.5 mol / L with a molar ratio of Fe:P of 1:0.6 Phosphoric acid solution, add sodium hydroxide solution to adjust the pH to 11, filter to obtain ferrous phosphate precipitate, and dry the ferrous phosphate precipitate at 150°C for 9 hours. Disperse ferrous phosphate in water, add a phosphoric acid solution with a molar ratio of Fe:P of 1:0.35, heat the mixed solution to 60°C, and stir for 2 hours; slowly add a molar ratio of Fe:Li to the mixed solution of 1:0.95 Lithium hydroxide solution, and stirred for 3 hours to obtain a suspension. Subsequently, glucose with a mass of 5% of the ferrous phosphate mass was dissolved in water, and slowly added to the suspension, stirred for 2 hours, and then spray-dried to obtain a lithium iron phosphate precursor. The lithium iron...
Embodiment 2
[0029] 1mol ferric chloride is dissolved in water, is configured as the iron ion solution that concentration is 2mol / L, adds the ascorbic acid that is 100% of iron ion molar percentage, then adds concentration is 1mol / L, and molar ratio Fe:P is 1:0.62 ammonium dihydrogen phosphate solution, and potassium hydroxide was added to adjust the pH value to 10, the ferrous phosphate precipitate was obtained by filtration, and the ferrous phosphate precipitate was dried at 150°C for 9 hours. Disperse ferrous phosphate in water, add a phosphoric acid solution with a molar ratio of Fe:P of 1:0.38, heat the mixed solution to 70°C, and stir for 3 hours; slowly add a molar ratio of Fe:Li to the mixed solution Lithium hydroxide solution, and stirred for 2 hours to obtain a suspension. Subsequently, sucrose whose mass is 10% of the mass of ferrous phosphate is dissolved in water, slowly added into the suspension, stirred for 3 hours, and then spray-dried to obtain a lithium iron phosphate pre...
Embodiment 3
[0031]1mol ferrous chloride is dissolved in water, is configured into the ferrous ion solution that concentration is 1.5mol / L, adds the ascorbic acid that is 3% of ferrous ion mole percentage, then adds concentration and is 1mol / L, molar ratio Fe:P Diammonium hydrogen phosphate solution of 1:0.7, adding sodium hydroxide to adjust the pH value to 11, filtering to obtain ferrous phosphate precipitate, and drying the ferrous phosphate precipitate at 170°C for 6 hours. Disperse ferrous phosphate in water, add a phosphoric acid solution with a molar ratio of Fe:P of 1:0.3, heat the mixed solution to 70°C, and stir for 3 hours; slowly add a molar ratio of Fe:Li to the mixed solution Lithium hydroxide solution, and stirred for 2 hours to obtain a suspension. Dextrin whose mass is 5% of the mass of ferrous phosphate is dissolved in water, and slowly added into the suspension. After stirring for 3 hours, the lithium iron phosphate precursor was obtained by spray drying. Lithium iron ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com