Preparation method and quality control method of traditional Chinese medicine preparation for treating bronchitis and bronchial asthma
A technology for bronchial asthma and bronchitis, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, measuring devices, etc., can solve problems such as difficult radical cure, hormone dependence of patients, and increased asthma mortality, and achieves reduction of processes and turnover, and The effect of high bioavailability and shortened production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Take the ten herbs in the original prescription, take Poria cocos (1 / 2 prescription amount), and grind them into fine powder; Ginkgo and bitter almonds are ground into the coarsest powder, and decoct the remaining seven flavors such as Poria cocos and earthworm for three times. The first and second times are 2 hours each, and the third time is 1 hour. Add 10 times the amount of water each time, combine the decoction, filter, and concentrate the filtrate under reduced pressure to a thick paste with a relative density of 1.16-1.18 (50°C). Add Poria cocos Fine powder, mixed, that is, the extraction extract.
Embodiment 2
[0032] Take the ten herbs in the original prescription, take 1 / 2 prescription amount of Poria cocos, and crush them into fine powder; Ginkgo and bitter almonds are crushed into the coarsest powder, and decoct with the remaining seven flavors such as Poria cocos and earthworm for three times. The second time is 2 hours each, and the third time is 1 hour. The amount of water added is 10 times, 8 times, and 8 times respectively. The decoction is combined and filtered. paste, add Poria cocos fine powder and mix well to get the extract.
Embodiment 3
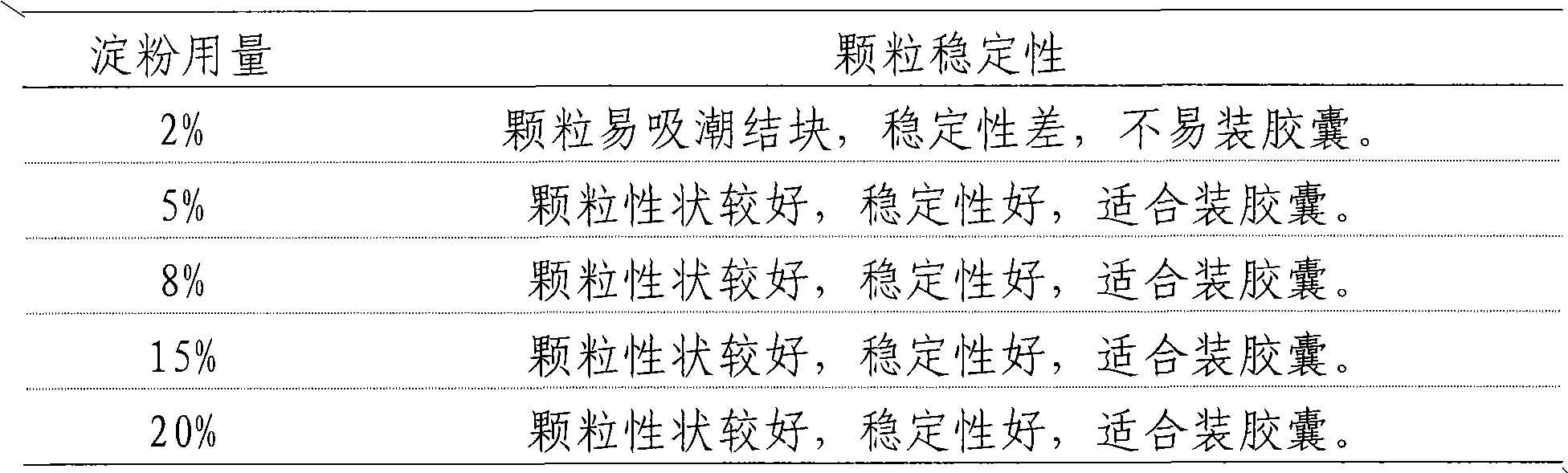
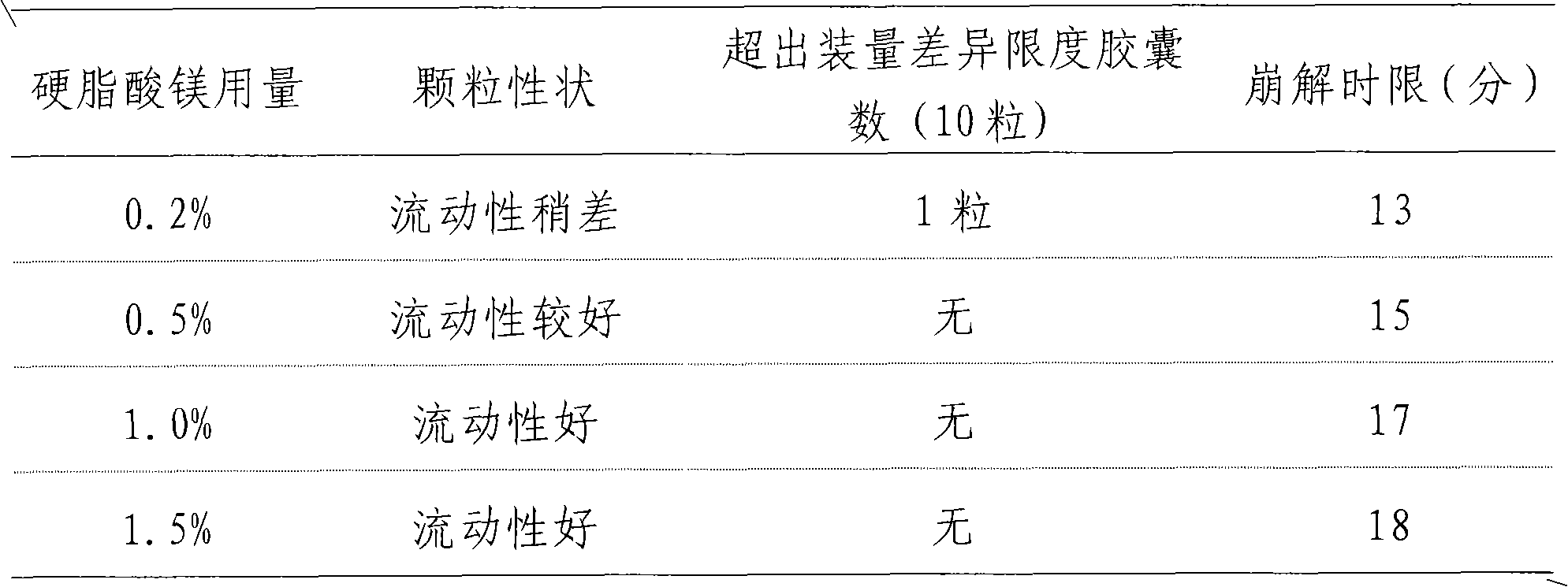
[0034] Add 80g of dextrin to any one of the above-mentioned extracts, mix evenly, and granulate in one step, add 3.5g sodium lauryl sulfate to the obtained granules, mix evenly, and pack into capsules, which is the capsule of the present invention agent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com