Strontium aluminate luminous material and controllable synthesis method thereof
A technology of luminescent materials and synthesis methods, which is applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problems affecting the shape and characteristics of materials, and achieve the effects of low cost, simple method and energy saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
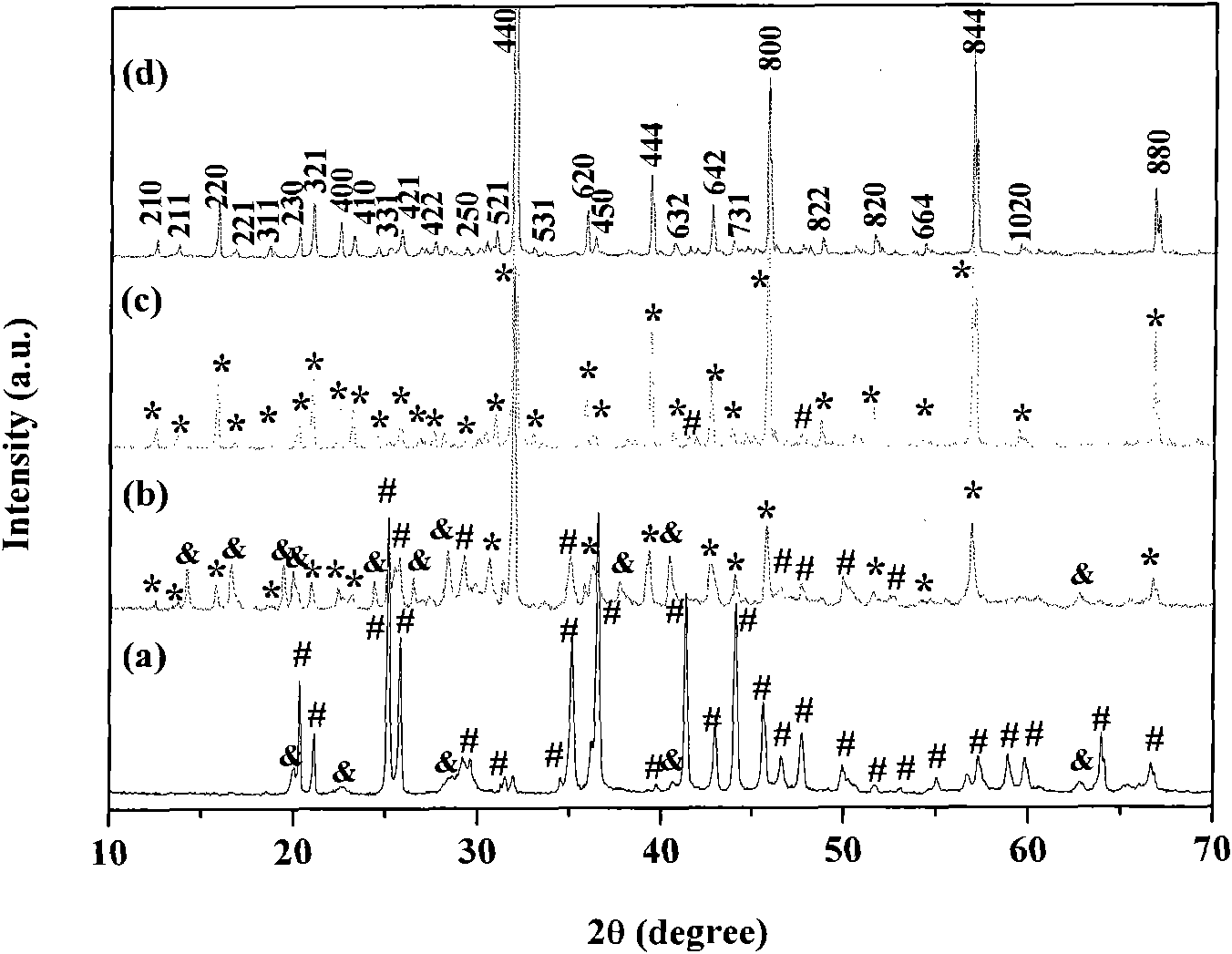
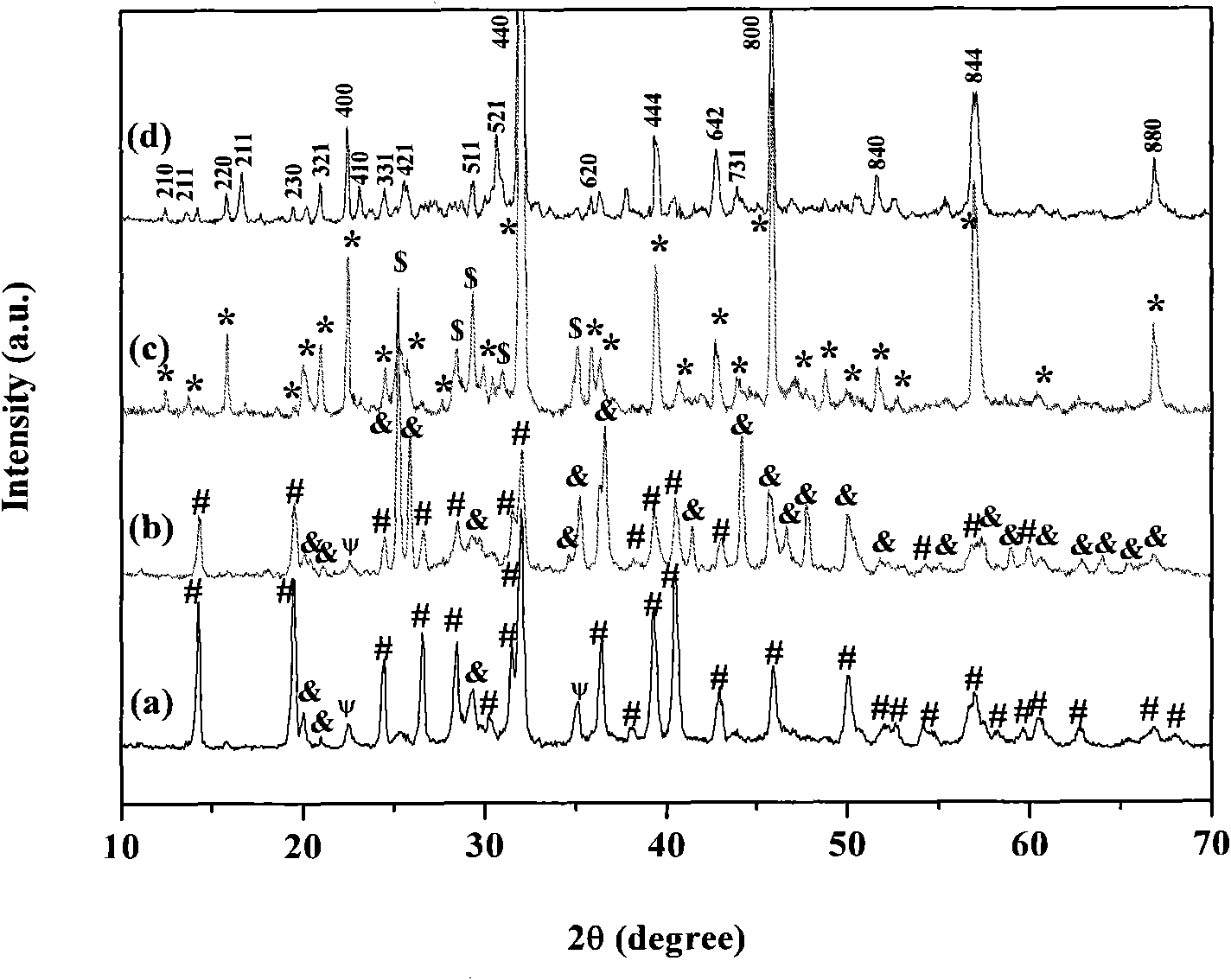
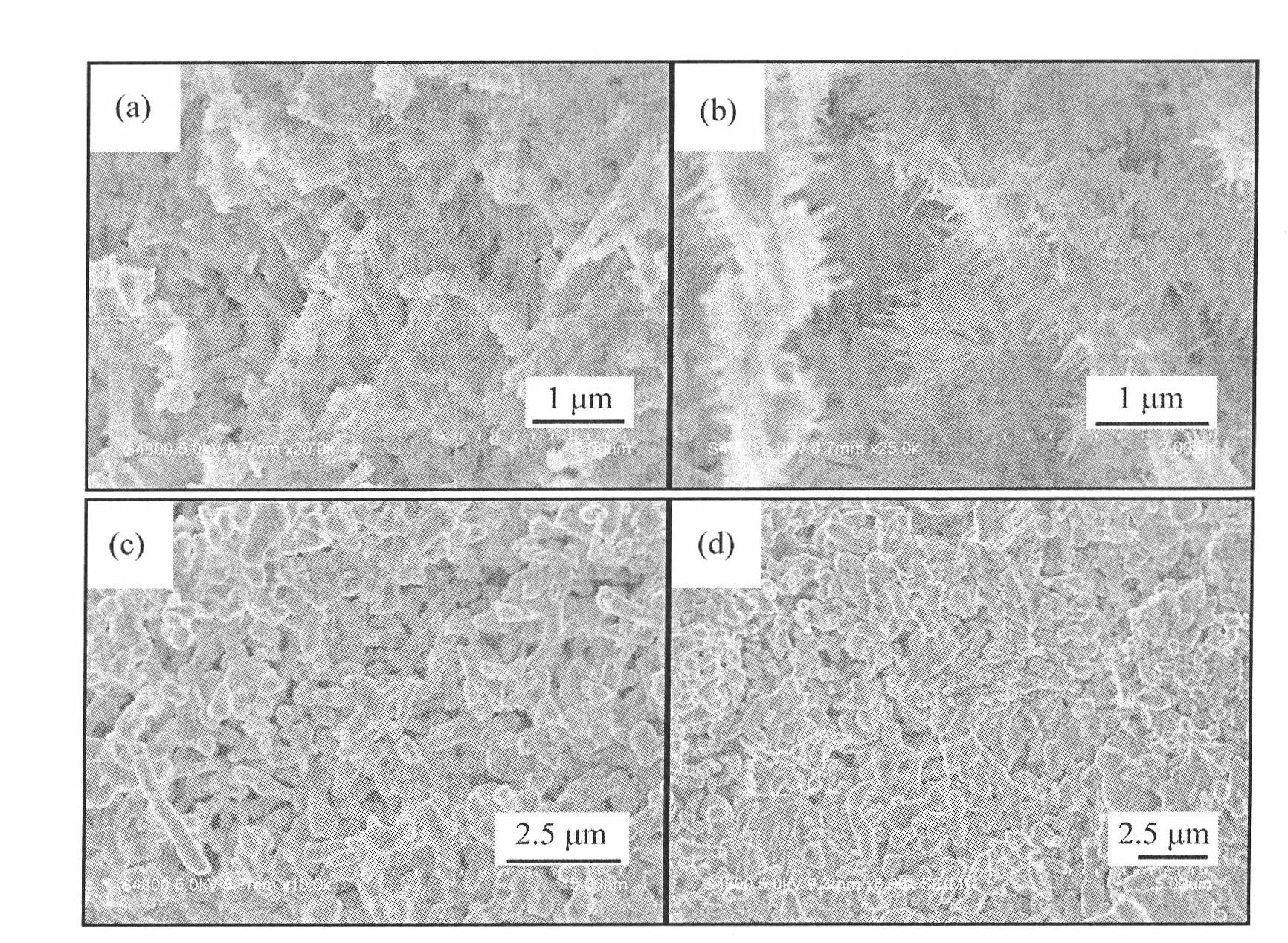
[0028] Embodiment 1: the preparation steps are as follows, 1), 0.006 mole aluminum nitrate Al(NO 3 ) 3 9H 2 O, 0.009 mole strontium nitrate Sr(NO 3 ) 2 , 0.00002 mole europium nitrate Eu(NO 3 ) 3 , 0.00003 mole dysprosium nitrate Dy(NO 3 ) 3 , 0.02 mole urea CO(NH 2 ) 2Put it in a beaker, add 40-50 ml of distilled water to it, stir to dissolve, put the above solution in a closed reaction kettle, and keep it warm at 120-200°C for 10-14 hours to obtain a white product. 2) The cooled white product is filtered and washed with distilled water and ethanol, and dried at 50-70° C. for 5-6 hours to obtain a precursor. 3) Anneal the precursor in an air atmosphere at 900°C for 2 to 4 hours to obtain the following: figure 1 (a), image 3 (a) The strontium aluminate luminescent material shown.
Embodiment 2
[0029] Embodiment 2: the preparation steps are as follows, 1), 0.006 mole aluminum nitrate Al(NO 3 ) 3 9H 2 O, 0.009 mole strontium nitrate Sr(NO 3 ) 2 , 0.00002 mole europium nitrate Eu(NO 3 ) 3 , 0.00003 mole dysprosium nitrate Dy(NO 3 ) 3 , 0.02 mole urea CO(NH 2 ) 2 Put it in a beaker, add 40-60 ml of distilled water to it, stir to dissolve, put the above solution in a closed reaction kettle, and keep it warm at 120-200°C for 10-14 hours to obtain a white product. 2) The cooled white product is filtered and washed with distilled water and ethanol, and dried at 50-70° C. for 5-6 hours to obtain a precursor. 3) Anneal the precursor in an air atmosphere at 1000°C for 2 to 4 hours to obtain the following: figure 1 (b), image 3 (b) The strontium aluminate luminescent material shown.
Embodiment 3
[0030] Embodiment 3: the preparation steps are as follows, 1), 0.006 mole aluminum nitrate Al(NO 3 ) 3 9H 2 O, 0.009 mole strontium nitrate Sr(NO 3 ) 2 , 0.00002 mole europium nitrate Eu(NO 3 ) 3 , 0.00003 mole dysprosium nitrate Dy(NO 3 ) 3 , 0.02 mole urea CO(NH 2 ) 2 Put it in a beaker, add 40-50 ml of distilled water to it, stir to dissolve, put the above solution in a closed reaction kettle, and keep it warm at 120-200°C for 10-14 hours to obtain a white product. 2) The cooled white product is filtered and washed with distilled water and ethanol, and dried at 50-70° C. for 5-6 hours to obtain a precursor. 3) Anneal the precursor in an air atmosphere at 1100°C for 2 to 4 hours to obtain the following: figure 1 (c), image 3 The strontium aluminate luminescent material shown in (c).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com