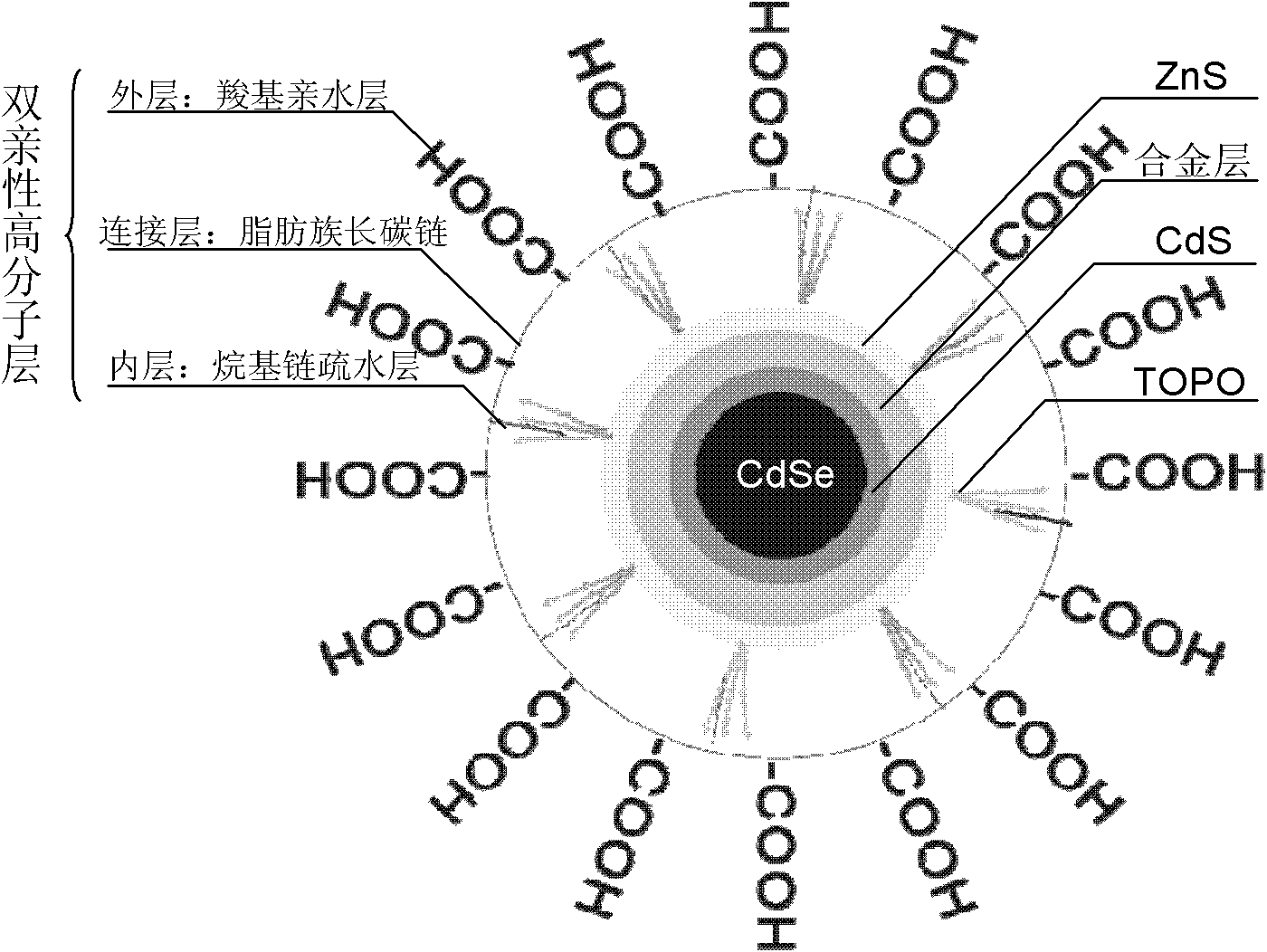
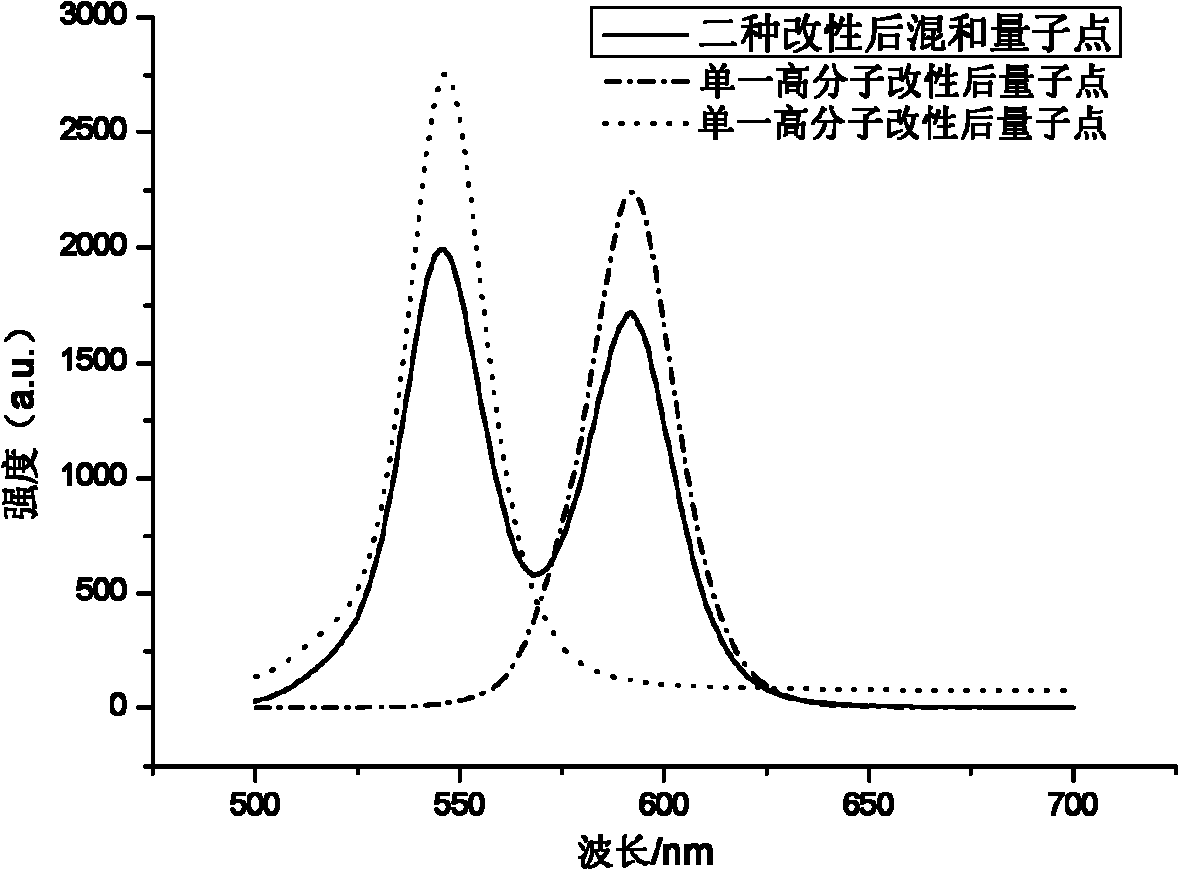
Amphiphilic macromolecular modified oil-soluble nuclear/shell quantum dots and preparation method
An amphiphilic, high-molecular technology, applied in the field of biochemical multi-throughput detection and nanomaterial preparation, can solve the problems of non-specific bonding, destroying the surface structure of quantum dots, and low yield, so as to achieve reduced toxicity, stable fluorescence performance, The effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Take 1 mL of chloroform solution of CdSe nuclear quantum dots, add 3 mL of octadecene, and take 0.1 g of octadecylamine and tri-n-octyl phosphorus oxide in a flask; after 30 minutes of argon gas, heat to 100 ° C, After keeping for 8 minutes, continue to heat up to 200°C; calculate the Cd required to prepare each layer of quantum dots 2+ , Zn 2+ , S 2- The amount, dropwise added the special Cd, Zn, S shell precursor solution; prepared CdSe / CdS / Cd 0.4 Zn 0.6 S / ZnS quantum dots were centrifuged and purified twice with acetone, and then stored in a sealed, dark place at low temperature.
[0028] (2) Poly-tert-butylacrylic acid, hexylamine and coupling agent EDC.HCl are mixed and dissolved in dimethylformamide, wherein the molar ratio of poly-tert-butylacrylic acid to hexylamine is 1:20, coupling agent and hexylamine The molar ratio was 1:1, and the reaction was carried out for 8 hours to obtain poly-tert-butylacrylic acid-hexylamine whose long chain was polyacrylic ...
Embodiment 2
[0033] (1) Take 1.75mL of CdSe nuclear quantum dot chloroform solution, add 3.75mL of octadecene, and take 0.2g of octadecylamine and tri-n-octyl phosphorus oxide in the flask; ℃, after keeping for 9min, continue to heat up to 210℃; calculate the Cd needed to prepare each layer of quantum dots 2+ , Zn 2+ , S 2- The amount, dropwise added the special Cd, Zn, S shell precursor solution; prepared CdSe / CdS / Cd 0.4 Zn 0.6 S / ZnS quantum dots were centrifuged and purified twice with acetone, and then stored in a sealed, dark place at low temperature.
[0034] (2) Polyethylacrylic acid, heptylamine and coupling agent EDC.HCl are mixed and dissolved in dimethylformamide, wherein the molar ratio of polyethylacrylic acid to heptylamine is 1:30, and the molar ratio of coupling agent to heptylamine 1.25:1, and reacted for 10 hours to obtain polyethylacrylic acid-heptylamine whose long chain is polyacrylic acid homopolymer A.
[0035] (3) CdSe / CdS / Cd 0.4 Zn 0.6 S / ZnS core / shell quantu...
Embodiment 3
[0039] (1) Take 2.25mL of CdSe nuclear quantum dot chloroform solution, add 4mL of octadecene, and take 0.25g of octadecylamine and tri-n-octylphosphorus oxide in the flask; after argon gas for 35min, heat to 100°C , after keeping for 9min, continue to heat up to 220°C; calculate the Cd needed to prepare each layer of quantum dots 2+ , Zn 2+ , S 2- The amount, dropwise added the special Cd, Zn, S shell precursor solution; prepared CdSe / CdS / Cd 0.4 Zn 0.6 S / ZnS quantum dots were centrifuged and purified twice with acetone, and then stored in a sealed, dark place at low temperature.
[0040] (2) Polymethacrylic acid, octylamine and coupling agent EDC.HCl are mixed and dissolved in dimethylformamide, wherein the molar ratio of polymethacrylic acid to octylamine is 1:50, and the molar ratio of coupling agent to octylamine 1.75:1, and reacted for 10 hours to obtain polymethacrylic acid-octylamine whose long chain is polyacrylic acid homopolymer A.
[0041] (3) CdSe / CdS / Cd 0.4 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com