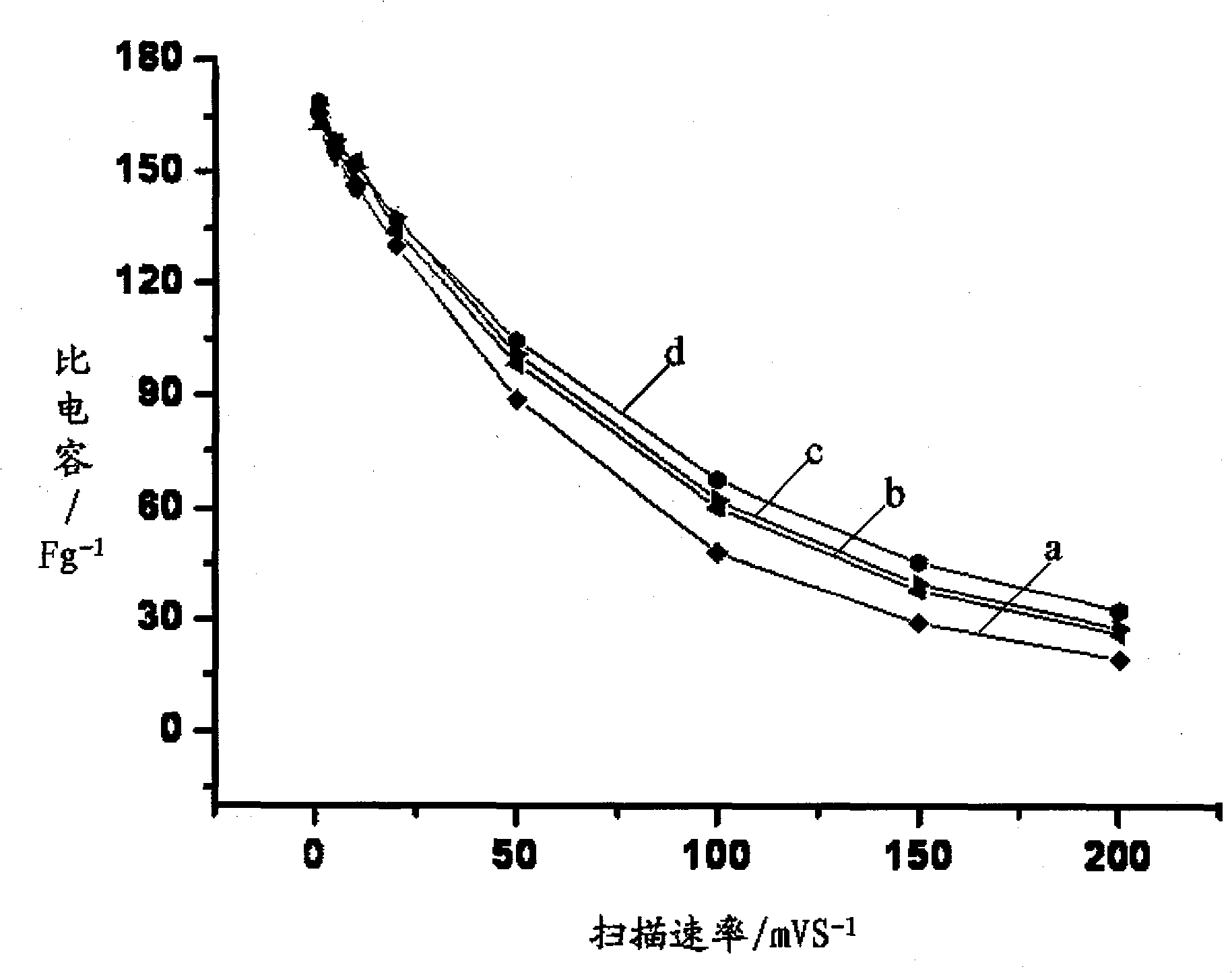
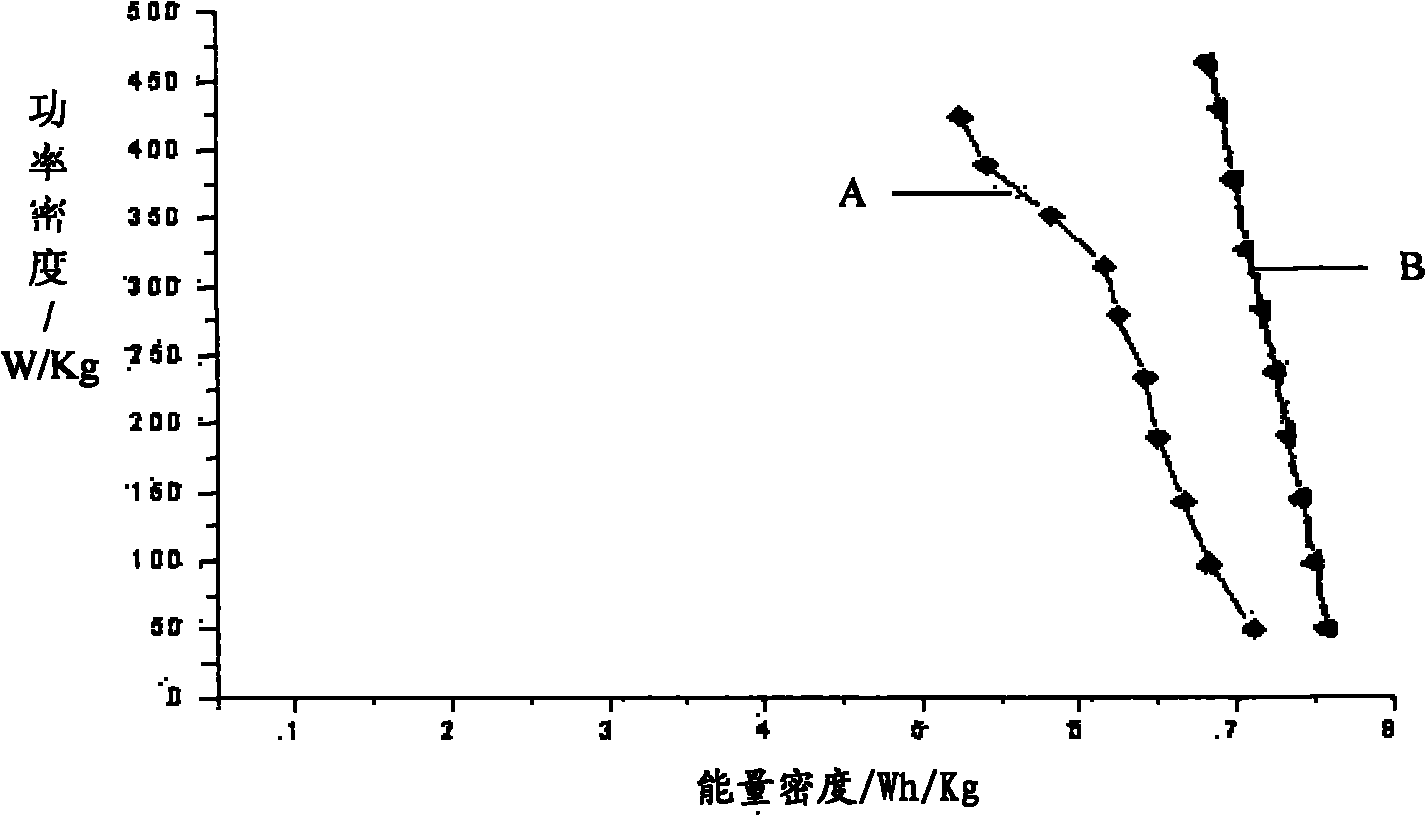
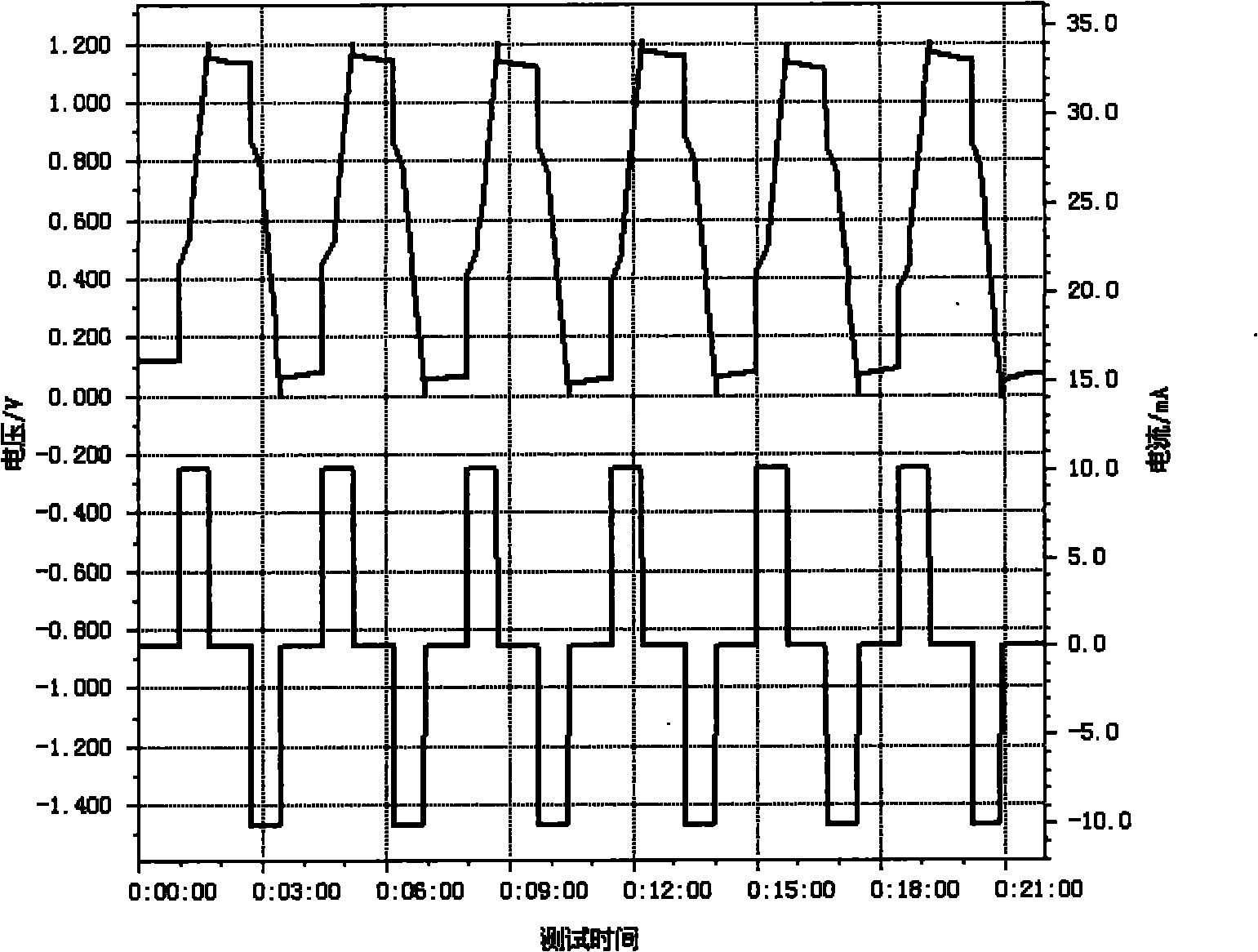
Electrolyte for super capacitor and super capacitor
A supercapacitor and electrolyte technology, applied in the field of electrochemistry, can solve the problems affecting the performance of capacitors, low solubility, low electrolyte, etc., and achieve the effects of reducing production cost and use cost, simple preparation process, and slow capacity decay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Dissolve 280.98g of commercially available sodium perchlorate in 1L of deionized water to obtain an electrolyte solution with a concentration of 2M;
[0041] Use the DJS~10C conductivity meter to measure the conductivity of the above electrolyte at room temperature, which is 218mScm -1 ;
[0042] Using a PerkinElmer differential thermal analyzer in the range of 25°C to -70°C, the freezing point of the above electrolytic solution was measured at a cooling rate of 1°C / min, and it was -26.23°C.
Embodiment 2
[0044] Dissolve 421.47g of commercially available sodium perchlorate in 1L of deionized water to obtain an electrolyte solution with a concentration of 3M;
[0045] Use the DJS~10C conductivity meter to measure the conductivity of the above electrolyte at room temperature, which is 273mScm -1 ;
[0046] Using a PerkinElmer differential thermal analyzer in the range of 25°C to -70°C, the freezing point of the above electrolytic solution was measured at a cooling rate of 1°C / min, and it was -30.68°C.
Embodiment 3
[0048] Dissolve 561.96g of commercially available sodium perchlorate in 1L of deionized water to obtain an electrolyte solution with a concentration of 4M;
[0049]Use the DJS~10C conductivity meter to measure the conductivity of the above electrolyte at room temperature, which is 292mScm -1 ;
[0050] Using a PerkinElmer differential thermal analyzer in the range of 25°C to -70°C, the freezing point of the above electrolytic solution was measured at a cooling rate of 1°C / min, and it was -36.33°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com