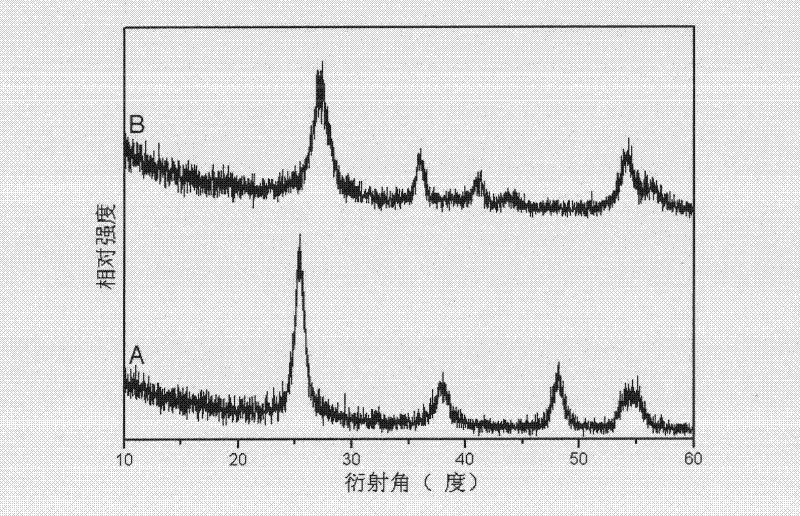
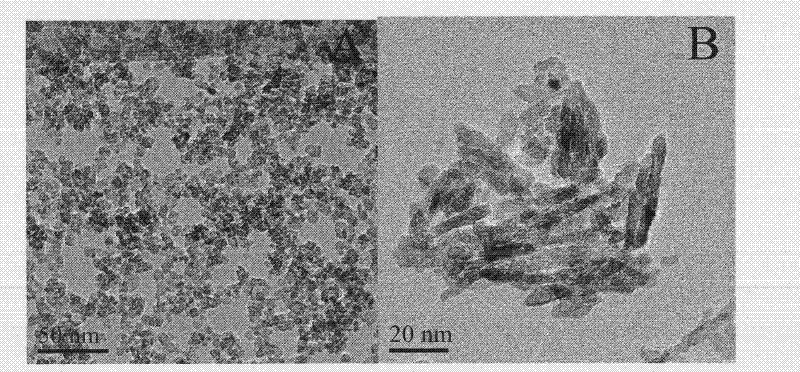
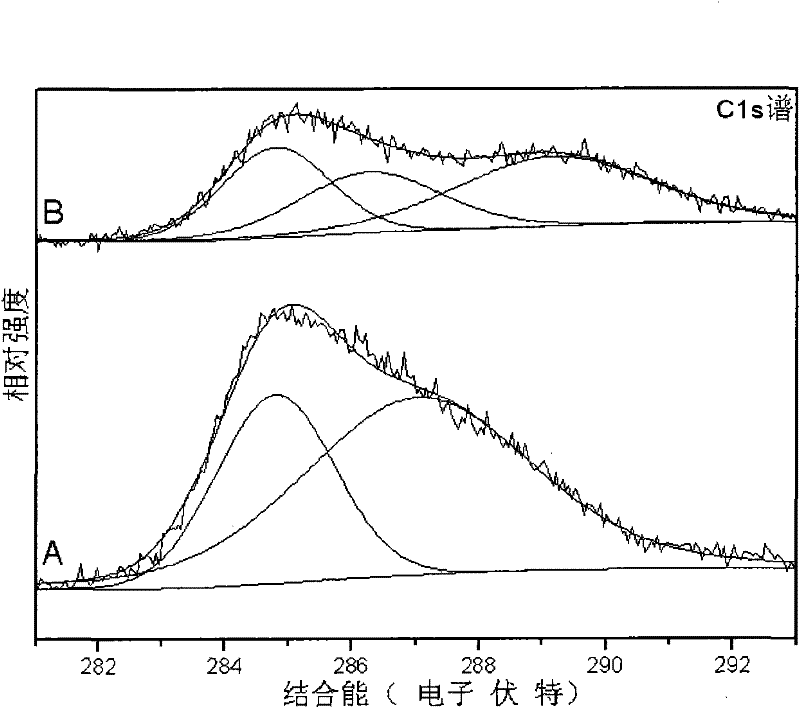
Preparation method of low-temperature non-aqueous sol-gel of high-activity carbon-chlorine codoped titanium dioxide visible light catalyst
A titanium dioxide, co-doping technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., to achieve the effects of low synthesis temperature, easy operation, and small industrial amplification factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Carbon and chlorine co-doped anatase titanium dioxide preparation, the preparation steps are:
[0031] Step 1: Add isopropyl titanate to a mixed solvent of ethanol and monochloromethane, the volume of the mixed solvent is 15 milliliters, the volume ratio of ethanol to monochloromethane is 5:1, and the amount of isopropyl titanate is 0.01 mole / liter;
[0032] Step 2: subjecting the mixed solution to solvent heat treatment at a temperature of 120° C. for 12 hours;
[0033] Step 3: After step 2 is completed, filter and rinse the filtered solid product with distilled water and ethanol four times respectively, and vacuum dry the product at 100° C. for 6 hours to obtain carbon and chlorine co-doped anatase titanium dioxide.
Embodiment 2
[0035] Carbon and chlorine co-doped anatase titanium dioxide preparation, the preparation steps are:
[0036] Step 1: add titanium tetrachloride in the mixed solvent of ethanol and monochloromethane, the volume of its mixed solvent is 15 milliliters, and the volume ratio of ethanol and monochloromethane is 4: 1, and the amount of titanium tetrachloride is 0.01 mol / Lift;
[0037] Step 2: subjecting the mixed solution to solvent heat treatment at a temperature of 100°C for 18 hours;
[0038] Step 3: After step 2 is completed, filter and rinse the filtered solid product three times with distilled water and ethanol respectively, and dry the product in vacuum at 100°C for 6 hours to obtain carbon and chlorine co-doped anatase titanium dioxide.
Embodiment 3
[0040] Carbon and chlorine co-doped anatase titanium dioxide preparation, the preparation steps are:
[0041]Step 1: Add tetrabutyl titanate to a mixed solvent of ethanol and monochloromethane, the volume of the mixed solvent is 15 milliliters, the volume ratio of ethanol to monochloromethane is 5:2, and the amount of tetrabutyl titanate is 0.01 mole / liter;
[0042] Step 2: subjecting the mixed solution to solvent heat treatment at a temperature of 100°C for 18 hours;
[0043] Step 3: After step 2 is completed, filter and rinse the filtered solid product three times with distilled water and ethanol respectively, and dry the product in vacuum at 100°C for 6 hours to obtain carbon and chlorine co-doped anatase titanium dioxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com