Alpha-galactosidase and expression and purification method thereof
A technology of galactosidase and expression vector, which is applied in the field of purification, bacterial α-galactosidase and its expression, and achieves the effects of simple expression and purification method, broad market prospect and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, cloning of α-galactosidase α-gal coding gene
[0048] Genomic DNA of Bacteriodes fragilis ATCC25285 (purchased from American TypeCulture Collection (ATCC)) was extracted using the Genomic DNA Extraction Kit of Promega Company according to the instructions. Using the extracted genomic DNA as a template, use an upstream primer (sequence: 5′-GC GGATCCATGAAGACAATCCTACTCTT-3′) and a downstream primer (sequence:
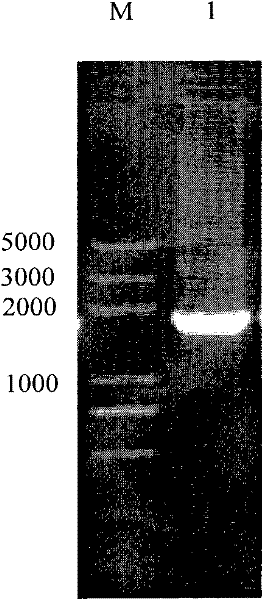
[0049] 5'GGAAGCTTTCGCTCTGAAATCTTCACGT-3') for PCR amplification. The PCR conditions were as follows: pre-denaturation at 95°C for 5 min; then denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 120 s, a total of 30 cycles; finally, extension at 72°C for 10 min. After the reaction, the PCR product was detected by 1% agarose gel electrophoresis, and the results were as follows: figure 1 As shown (M: DL2000 DNA Marker, l: full-length gene of α-gal), a band with a molecular weight of about 1788bp was obtained, which was ...
Embodiment 2
[0052] Example 2, Expression and purification of α-galactosidase α-gal
[0053] 1. Construction of the expression vector pET-22b(+)-α-gal containing the coding sequence of the α-galactosidase α-gal gene
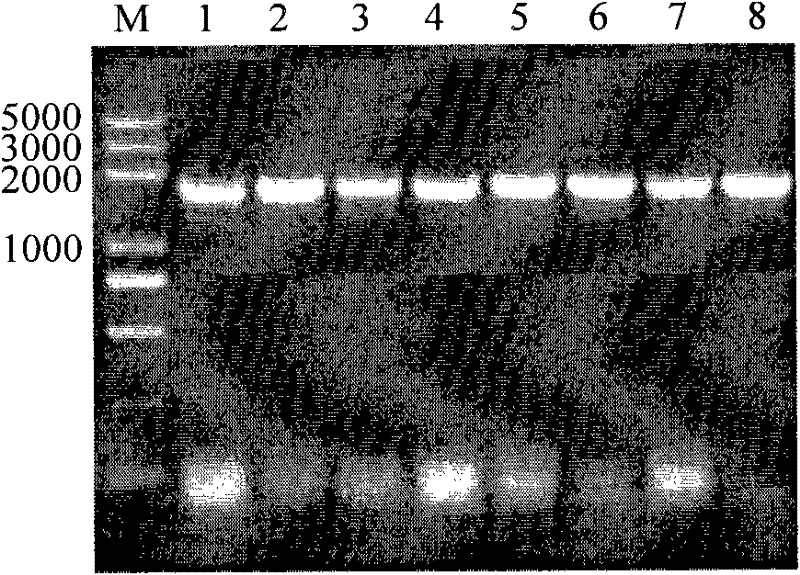
[0054] Using pGEM-α-gal as a template, use upstream primer (sequence: 5'-GGGGATCCTGATGACGACGACAAGGAGCGTGTTTATGACATT-3' (with BamH I restriction site), and downstream primer (sequence: 5'-GGAAGCTTTCGCTCTGAAATCTTCACGT-3' (with Hind III restriction site)) PCR amplification of α-galactosidase α-gal gene coding sequence. PCR conditions are: first 95 ° C pre-denaturation 5min; then 95 ° C denaturation 30s, 56 ° C annealing 30s, 72 ° C extension 120s, A total of 30 cycles; the last extension was 10min at 72°C. After the reaction, the PCR product was detected by 1% agarose gel electrophoresis, and the detection results were as follows: figure 2 As shown (M: DL2000DNA Marker, l: α-gal gene coding sequence), a band with a fraction of about 1700bp was obtained, which was consistent wi...
Embodiment 3
[0066] Example 3. Human erythrocytes are converted from type B to type O with α-galactosidase α-gal
[0067] Human B-type erythrocytes (provided by the Blood Transfusion Department of 307 Hospital) were washed twice with raw saline, and human B-type erythrocytes were washed twice with enzymatic hydrolysis buffer (isotonic citrate disodium hydrogen phosphate buffer, pH6.8), Take 0.2mL of packed B-type red blood cells, add 50ug of α-galactosidase expressed and purified in Example 2, make up 0.5mL with enzymolysis buffer, incubate at 26°C for 2 hours, and shake continuously to make it evenly enzymolyzed . Blood types were detected with anti-B blood typing reagent (Changchun Bode Biotechnology Co., Ltd.) and primary-side cross-match test.
[0068] 1. Detection of red blood cell blood type with blood typing reagent
[0069] Take 40ul of erythrocytes before or after enzymolysis, wash them twice with normal saline, make a 5% cell suspension, and add 20ul of anti-B and anti-A antibo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com