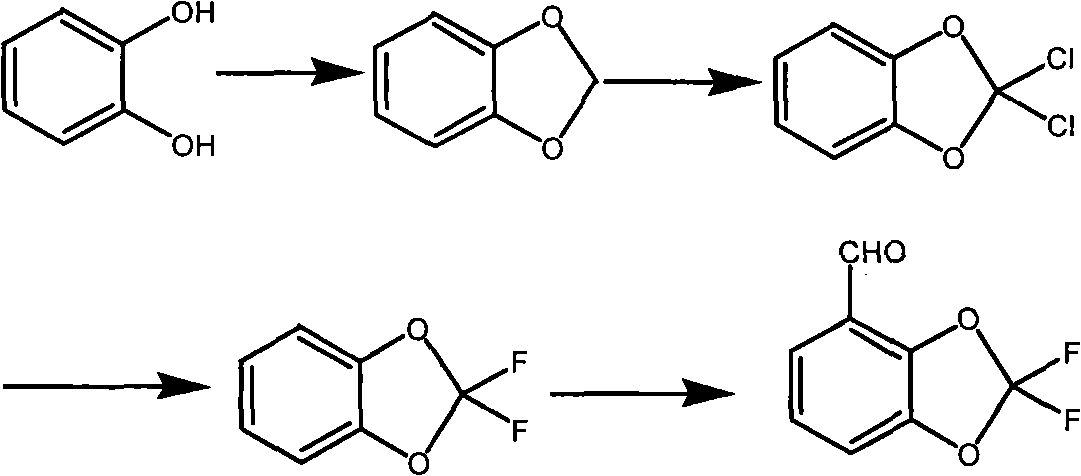
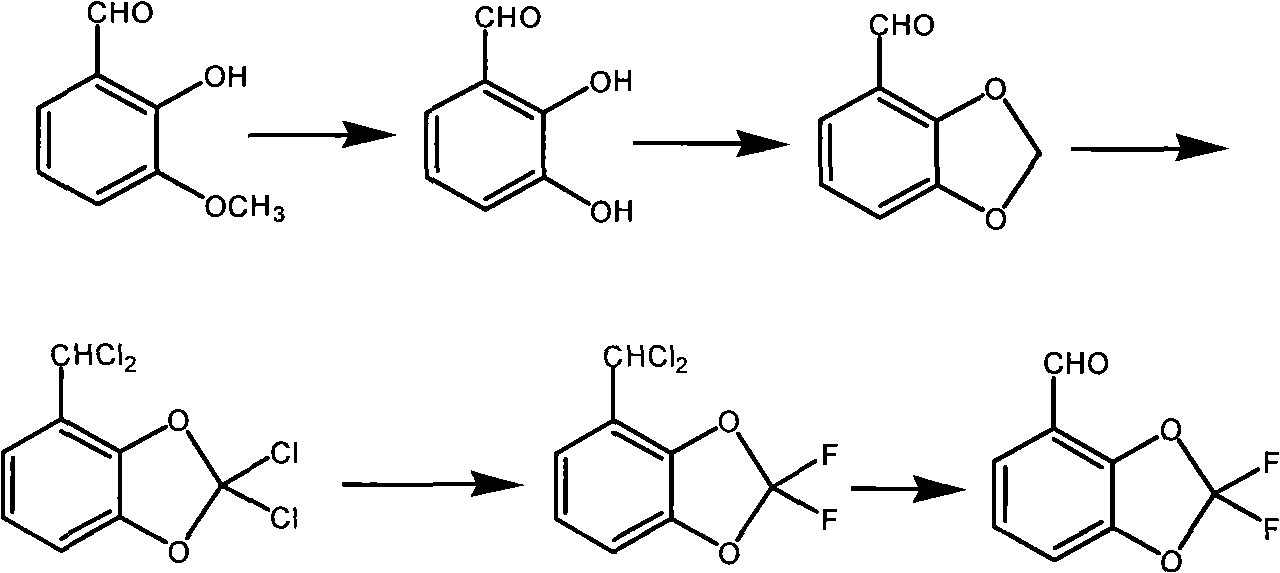
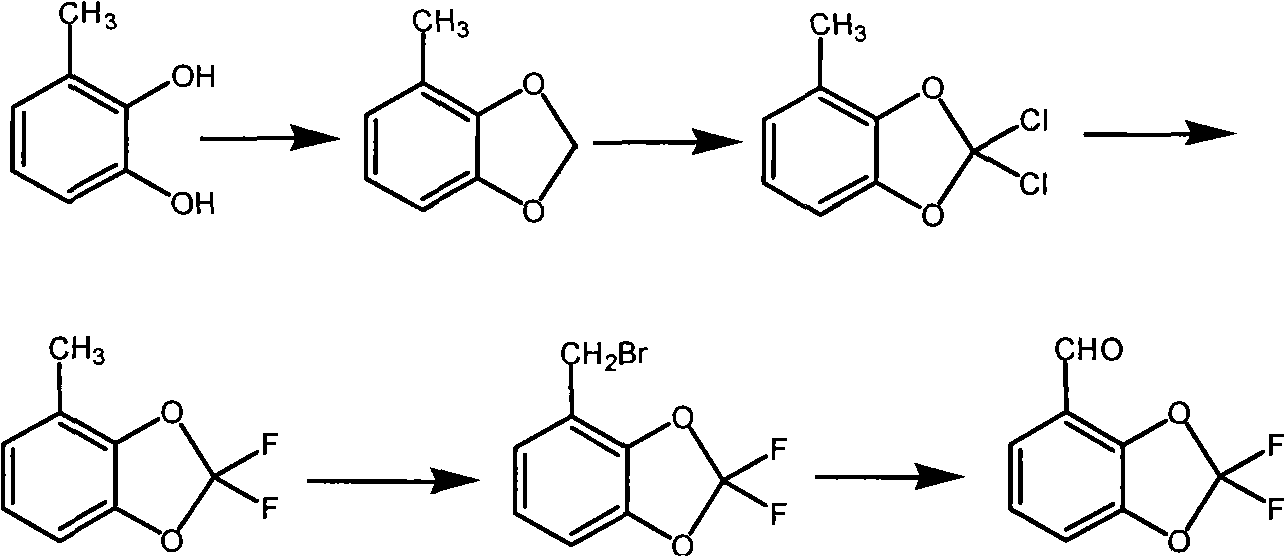
Method for synthesizing fludioxonil intermediate 4-aldehyde-2,2-difluorobenzodioxole
A synthesis method and intermediate technology, applied in the field of compound synthesis, can solve the problems of reducing the number of reaction steps and not fundamentally solving the problems of scarce raw materials, and achieve the effects of simplified operation, high economic value and social significance, and reduced production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of 3-methyl salicylaldehyde
[0031]
[0032] Put 5.76g of magnesium chips and 57.6g of anhydrous methanol into a 500ml four-neck flask, heat to reflux, and a large number of bubbles are formed. When the solution becomes turbid, add 50ml of toluene and continue to reflux. When the magnesium chips basically disappear, add toluene 50ml, continued to reflux for half an hour, put in 200ml of toluene, added dropwise 21.6g of o-cresol, heated up and distilled methanol until the temperature of the reactant reached 95°C. Keep at 95°C, add 24g of polyoxymethylene in batches, add 1 hour, distill off the generated methanol at the same time, continue to keep warm for 1 hour after the addition, track the plate, cool down after the reaction is complete, add 10% hydrochloric acid 175±10 gram, stirred, separated into layers, the toluene layer was washed twice with water, and desolvated under reduced pressure to obtain 24 g of a red oily substance with a mass percenta...
Embodiment 2
[0046] (1) The preparation of 3-methyl salicylaldehyde is the same as in Example 1.
[0047] (2) 24g of the product from the previous step was dissolved by adding 200ml of ethanol, adding 10g of sodium carbonate, slowly adding 122g of hydrogen peroxide with a mass percent content of 5%, and kept at 30°C for 5 hours, and the product was directly transferred to a 1L autoclave without separation , add 100ml of dichloromethane, 100ml of DMF, 8g of sodium carbonate, and 2g of tetrabutylammonium bromide, seal the autoclave, heat to 110°C for 2 hours, cool to room temperature, and open the pressure relief valve to release the pressure. Then the autoclave was opened, and the reactant was distilled to remove the low-boiling solvent, and water was added for azeotropic distillation to obtain 12 g of a colorless oily liquid with a normalized purity of 97.5% and a yield of 54%.
[0048] (3) Dissolve 12 g of the product from the previous step in 50 ml of toluene, add 21 g of phosphorus pent...
Embodiment 3
[0052] (1) The preparation of 3-methyl salicylaldehyde is the same as in Example 1.
[0053] (2) Preparation of 4-methylbenzodioxol
[0054] Add 200ml of methanol to dissolve 24g of the product from the previous step, add 16.8g of sodium bicarbonate, slowly add 136g of hydrogen peroxide with a mass percentage of 5%, keep it at 40°C for 4 hours, and transfer the product directly into a 1L autoclave without separation. Add 100ml of dichloromethane, 100ml of DMF, 6.4g of NaOH, and 2g of tetrabutylammonium bromide, seal the autoclave, heat to 90°C for 3 hours, cool to room temperature, and open the pressure relief valve to release the pressure. Then the autoclave was opened, and the reactant was distilled to remove the low-boiling solvent, and water was added for azeotropic distillation to obtain 13.6 g of a colorless oily liquid, with a normalized purity of 98.5% and a yield of 62.5%.
[0055] (3) Preparation of 4-methyl-2,2-dichlorobenzodioxol
[0056] Dissolve 13.6 g of the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com