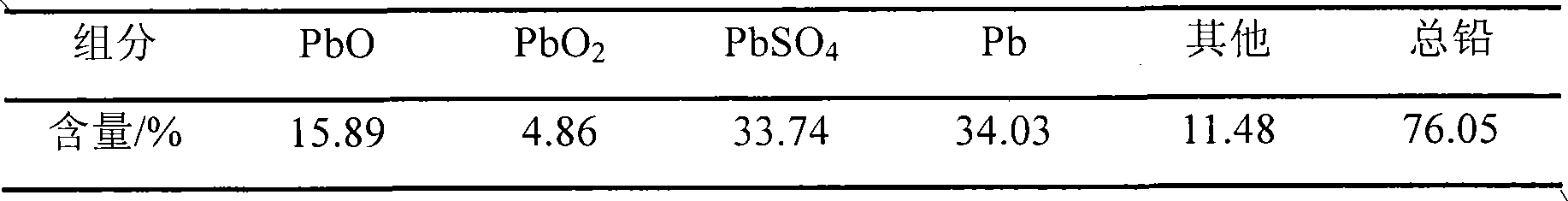
Method for preparing lead nitrate and lead oxide by using lead plaster of waste lead accumulator
A technology of waste lead storage battery and lead nitrate, which is applied in the field of producing lead nitrate and lead oxide from lead paste of waste lead storage battery. The effect of high product purity and low raw material consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: This embodiment is carried out according to the following steps:
[0031] 1. In the lead paste with a particle size of 74 μm, add the ammonium carbonate solution with a weight percent concentration of 6% in a weight ratio of 1:10, stir and react for 2 hours at a temperature of 95 ° C, filter, and wash the filter cake to nearly neutral. Collect filter cake, filtrate and washing solution for later use.
[0032] 2, in the above-mentioned lead paste desulfurization filter cake, it is 1: 10 to add the dilute nitric acid that the weight percent concentration is 7% and the weight ratio is that 1: 0.1 adds the hydrogen peroxide that the volume percent concentration is 30%, reacts at 60 ℃ After 1 hour, filter, the filter residue is sent to the desulfurization process, and the filtrate is collected for use.
[0033] 3. The filtrate obtained by leaching the nitric acid is evaporated and concentrated to a density of 1.32 g / cm3, adding the 70% concentrated nitric aci...
Embodiment 2
[0035] Embodiment 2: This embodiment is carried out according to the following steps:
[0036] 1. In the lead paste with a particle size of 74 μm, add the ammonium carbonate solution with a weight percent concentration of 5.5% in a weight ratio of 1:11, stir and react for 2.5 hours at a temperature of 80 ° C, filter, and wash the filter cake to nearly neutral. Collect filter cake, filtrate and washing solution for later use.
[0037] 2, in the above-mentioned lead paste desulfurization filter cake, it is 1: 9 to add the dilute nitric acid that the weight percentage concentration is 9% and the weight ratio is that 1: 0.15 adds the hydrogen peroxide that the volume percentage concentration is 29% by weight, reacts at 60 ℃ After 1 hour, filter, the filter residue is sent to the desulfurization process, and the filtrate is collected for use.
[0038] 3. The filtrate obtained by leaching the nitric acid was evaporated and concentrated to a density of 1.40g / cm 3 , adding 68% conc...
Embodiment 3
[0040] Embodiment 3: This embodiment is carried out according to the following steps:
[0041]1. In the lead paste with a particle size of 74 μm, add the ammonium carbonate solution with a weight percent concentration of 4.5% in a weight ratio of 1:12, stir and react for 2.5 hours under a temperature condition of 65 ° C, filter, and wash the filter cake to nearly neutral. Collect filter cake, filtrate and washing solution for later use.
[0042] 2, in the above-mentioned lead paste desulfurization filter cake, it is 1: 8.5 by weight to add the diluted nitric acid that the volume percent concentration is 11% and the weight ratio is that 1: 0.2 adds the hydrogen peroxide that the volume percent concentration is 28%, reacts at 45 ℃ 1.5 hours, filter, the filter residue is sent to the desulfurization process, and the filtrate is collected for use.
[0043] 3. The filtrate obtained by leaching the nitric acid was evaporated and concentrated to a density of 1.43g / cm 3 , under sti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com