Process for the manufacture of rutile titanium dioxide powders
A technology of titanium dioxide and rutile, applied in the direction of titanium dioxide, titanium oxide/hydroxide, nanotechnology for materials and surface science, etc., can solve problems that are not rutile
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
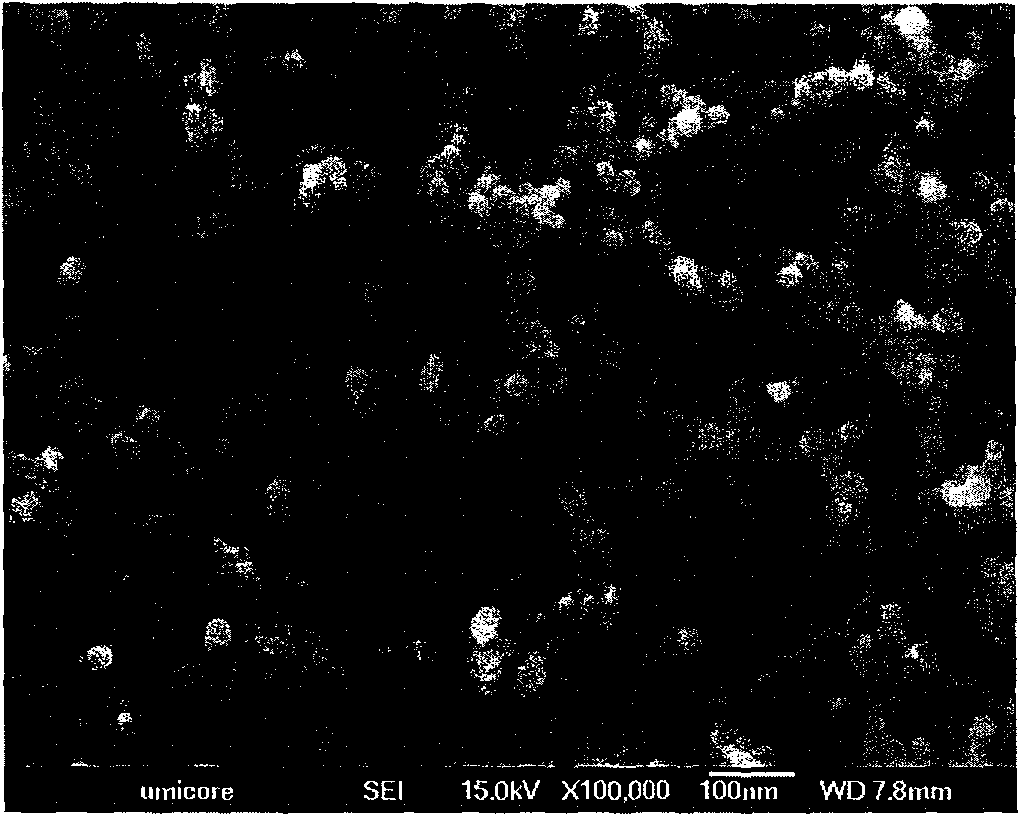
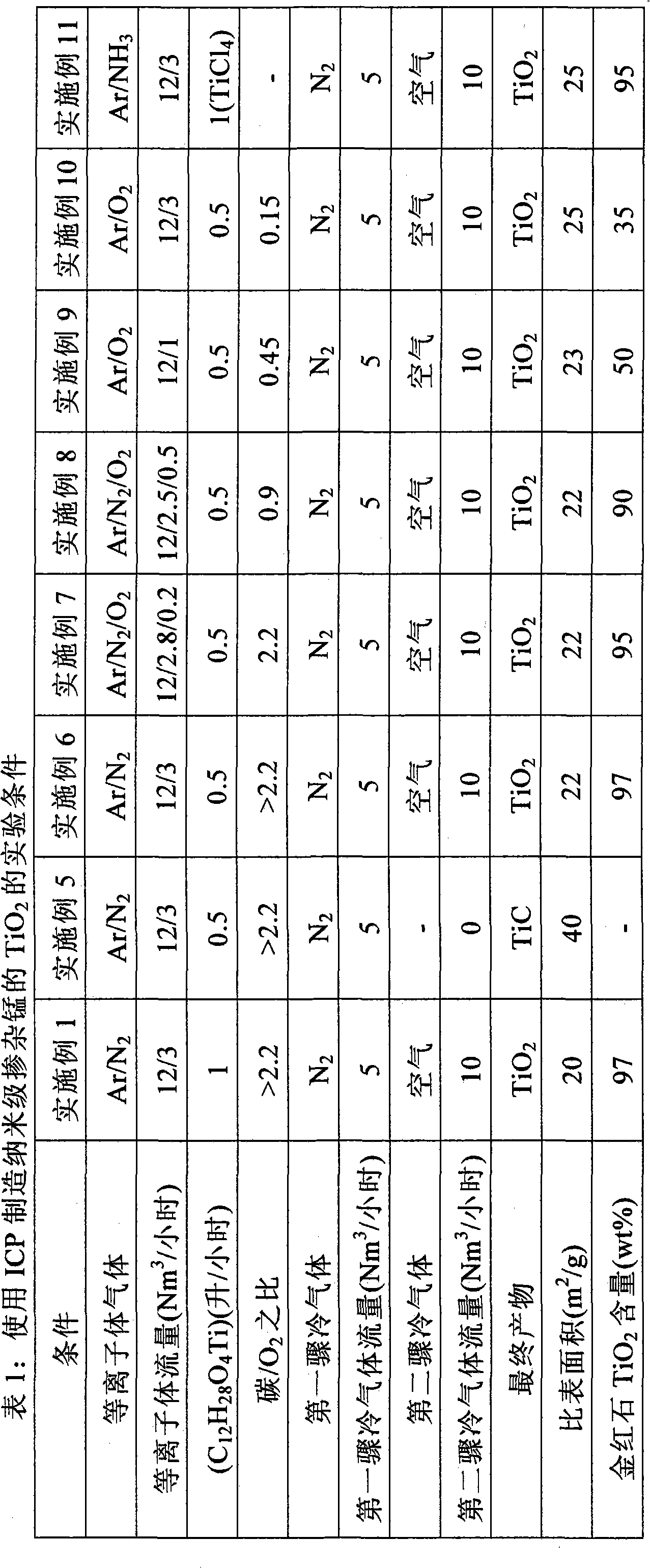
[0025] with 12Nm 3 / hr Argon and 3Nm 3 Argon / nitrogen plasma of nitrogen per hour, using a 25kW radio frequency (RF) inductively coupled plasma (ICP). The titanium isopropoxide is injected in the plasma at a flow rate of 1 liter / hour, resulting in a prevailing (i.e. in the reaction zone) temperature above 2000K in this first processing step, where the titanium isopropoxide is completely evaporated , so that it is easily nucleated to form cubic TiC nanopowders. 5Nm 3 A nitrogen flow per hour was used as the quench gas directly downstream of the reaction zone. This reduces the gas temperature to below 2000K. Furthermore, downstream, the 10Nm 3 Air per hour is blown into the airflow, thereby triggering a second processing step, which oxidizes the TiC powder to nanoscale rutile TiO 2 . Any residual carbon is also oxidized in this step. After filtration, nanoscale TiO is obtained 2 Powder with a rutile content of 97±2% and a specific surface area of 25±2m 2 / g. This co...
Embodiment 2
[0027] Under similar conditions, the apparatus of Example 1 was run. However, together with titanium isopropoxide, manganese isooctoate was injected into the plasma at a total injection flow rate of 1 liter / hour. After filtration, nanoscale manganese-doped TiO is obtained 2 Powder with a rutile content of 97±2% and a specific surface area of 25±2m 2 / g, the Mn content was 0.67±0.02%.
Embodiment 3
[0029] A 250kW direct current (DC) plasma torch was used with nitrogen as the plasma gas. The gas is at 150Nm 3 The plasma was discharged at a flow rate of / hour. A mixture of titanium isopropoxide and manganese isooctoate was injected downstream of the plasma at a flow rate of 25 kg / hour. In this step, the reactants were evaporated, resulting in a prevailing gas temperature of 2200K, and nucleated into Mn-doped TiC powder. Subsequently, in order to lower the gas temperature, 160Nm was applied 3 / hour nitrogen flow. Further downstream, at 6000Nm 3 Air is blown at a flow rate of / hour, thereby oxidizing TiC to nano-scale rutile TiO 2 . After filtration, a doped nanopowder was obtained with a rutile content of 97±2%, a Mn content of 0.67±0.02%, and a specific surface area of 18±2 m 2 / g, which corresponds to an average primary particle size of about 80 nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com