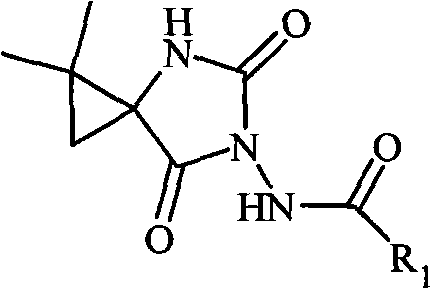
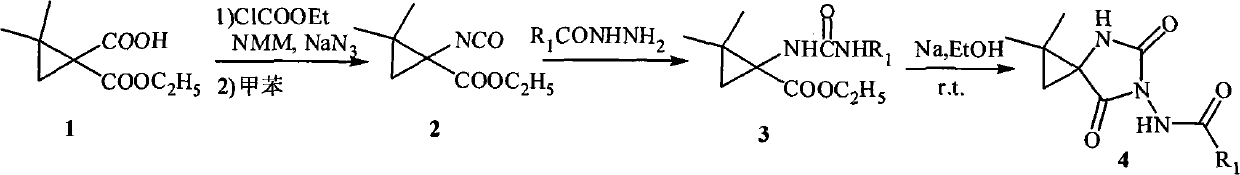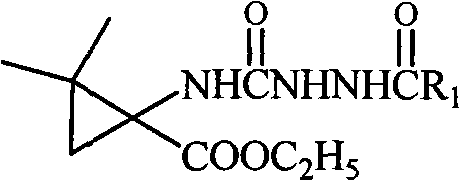N-3-aramid-5-cyclopropane spiro hydantoin derivative, preparation method and application thereof
A spirohydantoin and cyclopropane technology, applied in the field of N-3-arylamide-5-cyclopropane spirohydantoin derivatives and their preparation and application, can solve cognitive dysfunction, serious Allergic reactions, adverse reactions and other problems, to achieve the effect of simple preparation method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: 1-isocyanate-2, the synthesis of ethyl 2-dimethylcyclopropanecarboxylate
[0023] Dissolve ethyl 1-carboxy-2,2-dimethylcyclopropanecarboxylate (10mmol) in anhydrous tetrahydrofuran (30mL), cool in an ice-salt bath to about -15°C, and then add ethyl chloroformate (10mmol ) and N-methylpyrrolidone (NMM), immediately produced a white precipitate. At this temperature, after stirring the mixture for 20 minutes, the NaN 3 (10 mmol) in 5 mL of aqueous solution was added to the reaction solution, and stirring was continued for 1 hour. After the reaction was completed, a small amount of water was added to dissolve the insoluble matter, extracted with ethyl acetate, washed with saturated brine (2×10mL), washed with anhydrous Na 2 SO 4 Let dry overnight. After filtration, the solvent was evaporated under reduced pressure to obtain a light yellow liquid (note: compounds containing azide are explosive and cannot be evaporated to dryness). This crude product is tra...
Embodiment 2
[0026] Embodiment 2: 1-isocyanate-2, the synthesis of ethyl 2-dimethylcyclopropanecarboxylate
[0027] Dissolve ethyl 1-carboxy-2,2-dimethylcyclopropanecarboxylate (10mmol) in anhydrous tetrahydrofuran (30mL), cool in an ice-salt bath to about -5°C, and then add ethyl chloroformate (30mmol ) and N-methylpyrrolidone (NMM), immediately produced a white precipitate. After stirring the mixture for 30 minutes at this temperature, the NaN 3 (40 mmol) in 5 mL of aqueous solution was added to the reaction solution, and stirring was continued for 1 hour. After the reaction was completed, a small amount of water was added to dissolve the insoluble matter, extracted with ethyl acetate, washed with saturated brine (2×10mL), washed with anhydrous Na 2 SO 4 Let dry overnight. After filtration, the solvent was evaporated under reduced pressure to obtain a light yellow liquid (note: compounds containing azide are explosive and cannot be evaporated to dryness). This crude product is trans...
Embodiment 20
[0044] Example 20: Synthesis of 1,1-dimethyl-6-benzamide-4,6-diazaspiro[2.4]heptane-5,7-dione
[0045] Dissolve ethyl 2,2-dimethyl-1-benzohydrazide carboxamide cyclopropanecarboxylate (1 mmol) obtained in Example 1 in absolute ethanol (10 mL), then add metal Na, react at 5°C, TLC monitoring, after the completion of the reaction, ethanol was evaporated under reduced pressure, the residue was added a small amount of water, extracted with ethyl acetate (3 × 20mL), the organic layer was collected, washed with saturated brine, anhydrous Na 2 SO 4 Let dry overnight. Filtrate, evaporate the solvent under reduced pressure, and separate by silica gel column chromatography to obtain the compound 1,1-dimethyl-6-benzamide-4,6-diazaspiro[2.4]heptane-5,7-dione.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com