Short peptide and immunosuppressant containing the same and application thereof
An immunosuppressant and short peptide technology, which is applied in the field of medicine, can solve the problems of difficult production, high production cost, and high price, and achieve the effect of short structure, low side effects, and inhibition of binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Design and synthesis of embodiment 1 short peptide sequence
[0023] After sequence design, optimization and screening, 9 short peptides were obtained, as shown in Table 1. Sent to Shanghai Jill Biochemical Synthesis, the purity was >95%, and used for the experiments in the following examples.
[0024] Table 1. Short peptides obtained through design and optimization
[0025]
Embodiment 2
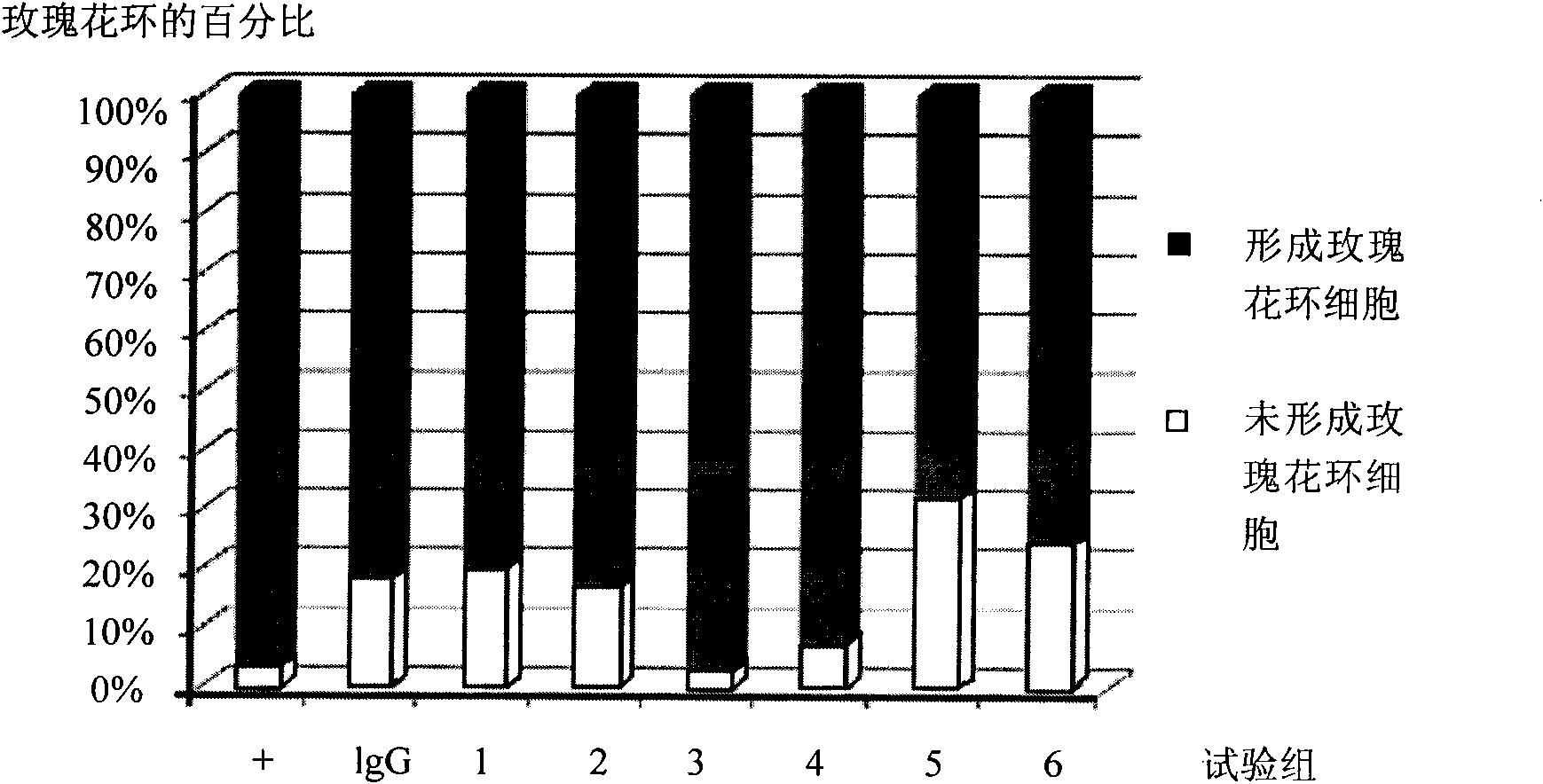
[0026]Example 2 The short peptide compound of the present invention specifically antagonizes the interaction between FcγRIIA and IgG
[0027] The short peptides used are: No. 1-5 short peptides.
[0028] (1) Cell culture
[0029] U937 cells (purchased from the Cell Bank of Shanghai Chinese Academy of Sciences), using RMPI1640 culture medium containing 10% (v / v) calf serum, at 37 ° C, 5% (v / v) CO 2 to cultivate. K562 cells (purchased from the Shanghai Chinese Academy of Sciences Cell Bank), using IMDM culture medium containing 10% (v / v) fetal bovine serum, 100 U / ml penicillin and 100 μg / ml streptomycin, at 37 ° C, 5% (v / v )CO 2 to cultivate.
[0030] (2) Determination of short peptide compound antagonizing FcγRIIA-IgG interaction activity
[0031] The binding of FcγR and IgG on the cell surface was counted by flow cytometry.
[0032] Specific steps: Take cells in the doubling phase, wash 3 times with sterile PBS, and resuspend to 1×10 6 / ml. In the negative control grou...
Embodiment 3
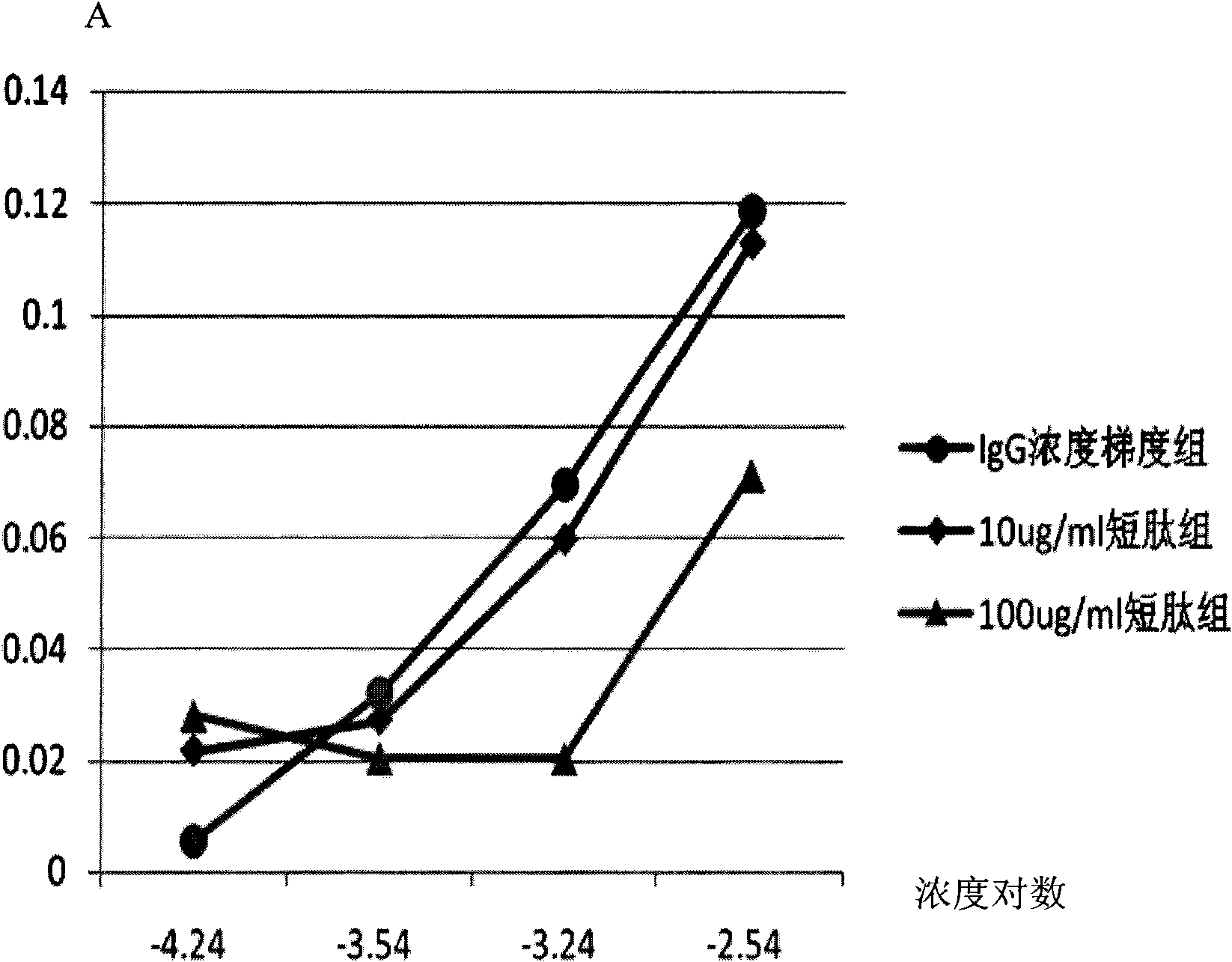
[0039] Example 3 The short peptide can antagonize the binding between FcγRIIA-IgG in a concentration-dependent manner
[0040] The short peptide used is peptide No. 1.
[0041] (1) Cell culture
[0042] K562 cells were cultured in IMDM medium containing 10% (v / v) fetal bovine serum, 100 U / ml penicillin and 100 μg / ml streptomycin at 37°C in 5% (v / v) CO 2 to cultivate.
[0043] (2) CELL ELISA to verify the concentration-dependent antagonism of short peptides between FcγRIIA-IgG
[0044] The 96-well plate was blocked with 2% (w / v) PBS-B (2% (w / v) PBS-B, dissolved in 100ml PBS with 2g of bovine serum albumin (BSA), similar to the following), 4 ℃ overnight or at room temperature for 2h. The K562 cells in the logarithmic growth phase were washed 3 times with 1% (w / v) PBS-B. Resuspend K562 cells in 1% (w / v) PBS-B, adjust the concentration to 6×10 6 / ml, add 100 μl of K562 cells to each well, and remove the supernatant after centrifugation. The IgG group was directly added with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com