Novel phosphorus series bisphenols and manufacture method of derivatives thereof
A technology of phenol derivatives and phosphorus compounds, applied in the field of manufacturing a series of derivatives, can solve the problems of high raw material cost, limited application, poor solubility and reactivity, etc., and achieve the effect of good heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
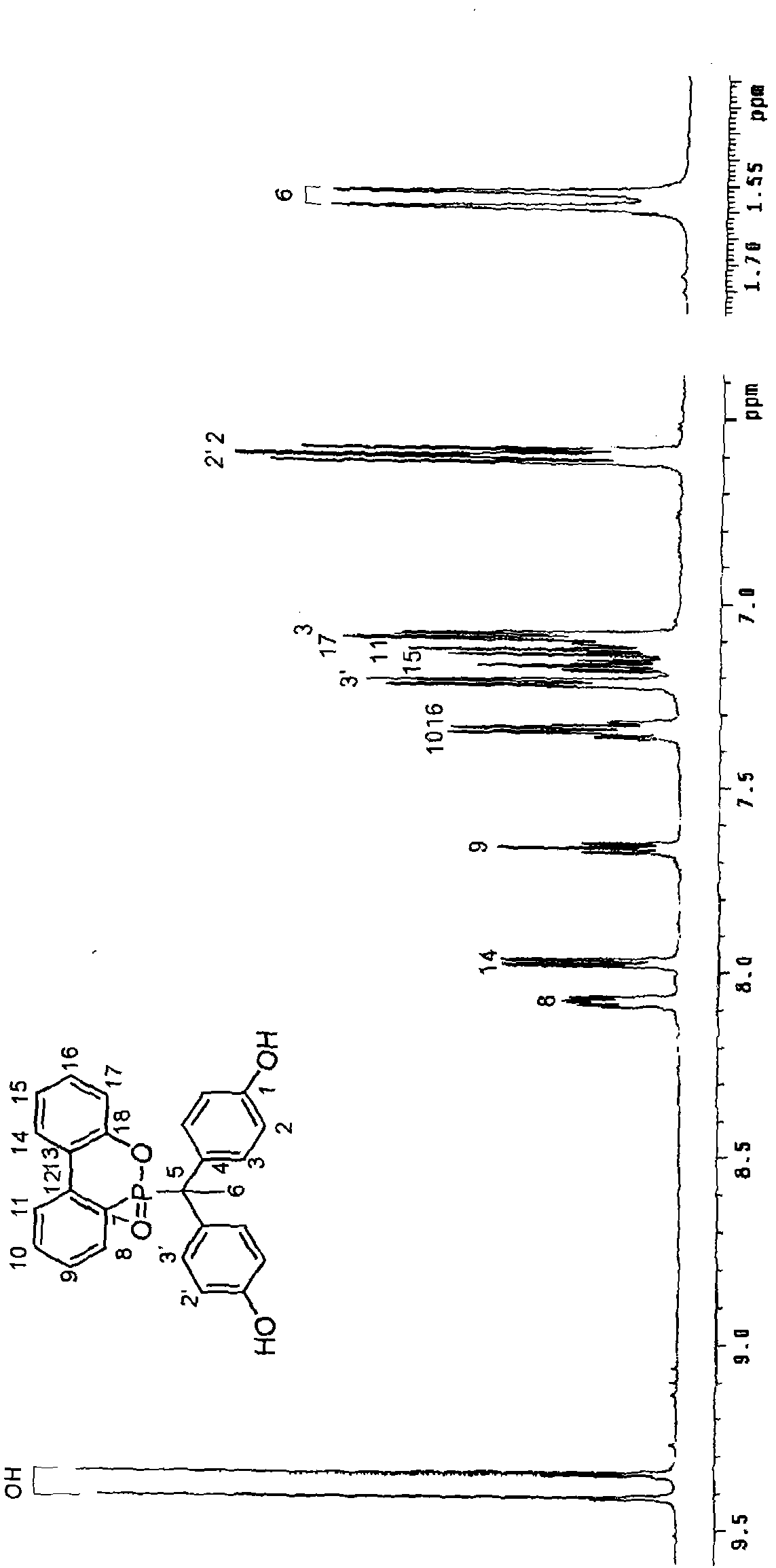
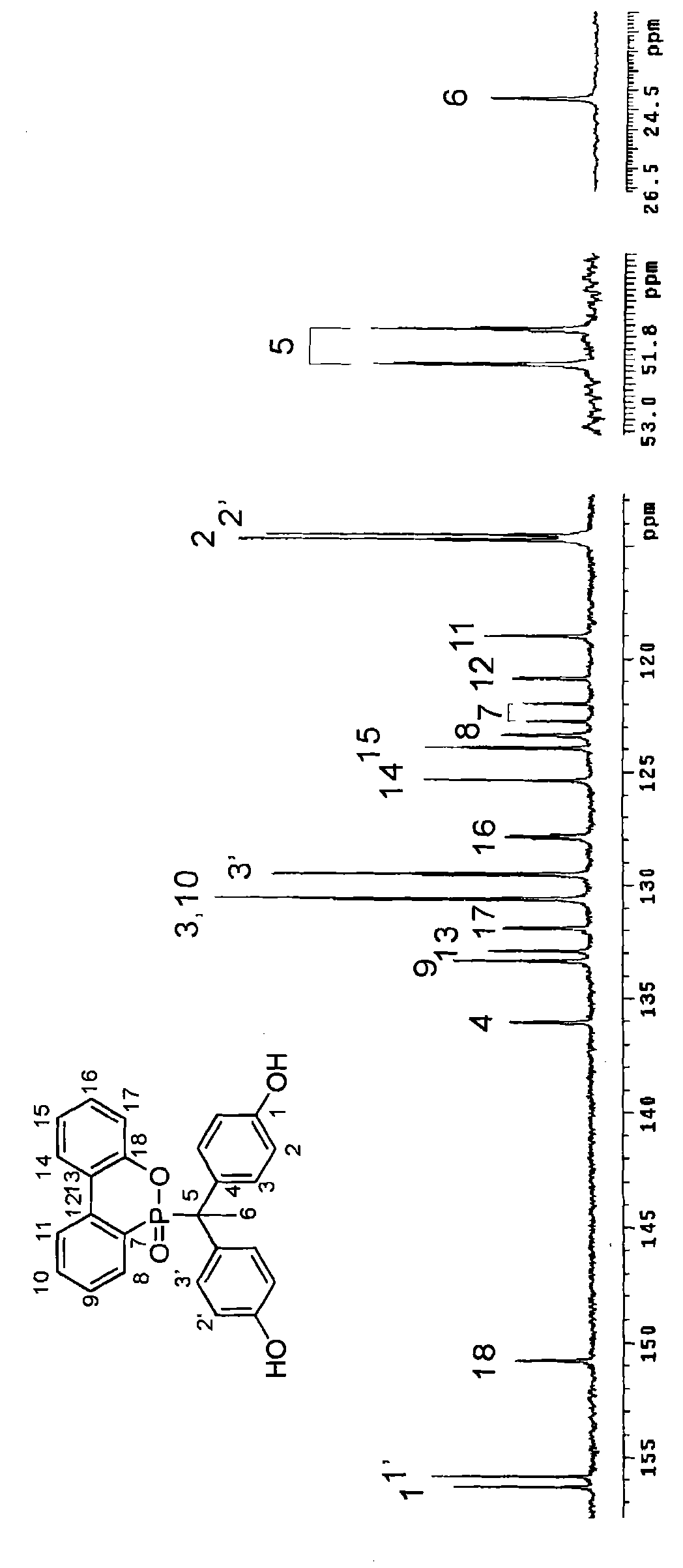
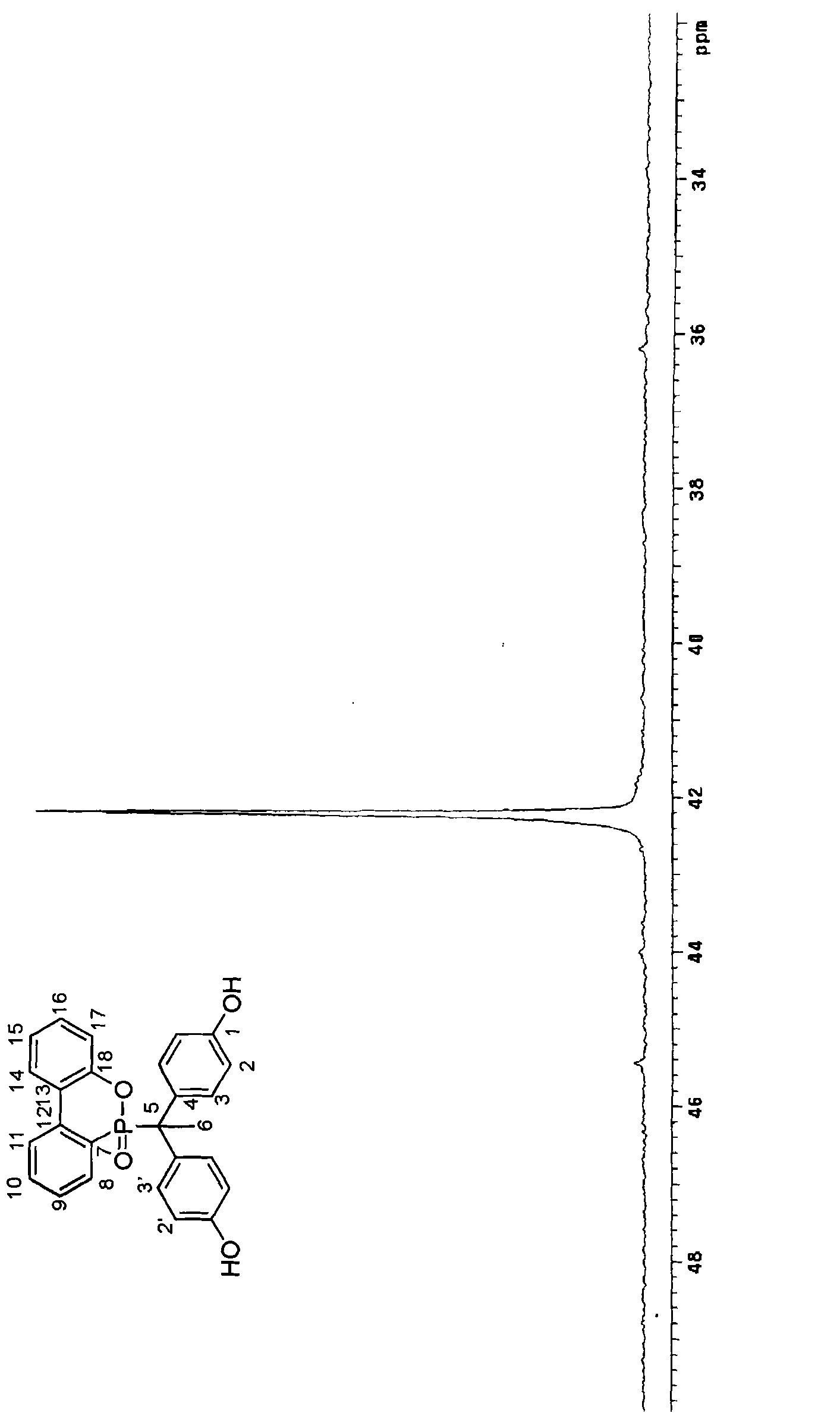
[0040] Synthesis Example 1: Synthesis of Compound A
[0041] According to an embodiment of the present invention, a phosphorus compound A in which R is methyl and A and B are OH is synthesized by using organic cyclic phosphorus compound DOPO, phenol, 4'-hydroxyacetophenone and an acid catalyst. Its synthetic steps are as follows:
[0042] The organic cyclic phosphorus compound DOPO (9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide) 10.81 g (0.05 mol), phenol 23.28 g (0.25 mol), 4'-hydroxy 6.81 g (0.05 mol) of acetophenone and 0.216 g of p-toluenesulfonic acid (2 wt% of DOPO) were placed in a 250 ml three-neck reactor.
[0043] Then, the reaction temperature was increased to 130° C., and the reaction was maintained for 24 hours, and then the stirring was stopped. The reactor was cooled to room temperature, dissolved in ethanol and poured into hot water to precipitate, filtered and dried to obtain product A with a yield of 85% and a melting point of 306°C.
[0044] Please...
Synthetic example 2
[0045] Synthesis Example 2: Synthesis of Compound A-cy
[0046] According to the embodiment of the present invention, A-cy is synthesized by using phosphorus compound A. Its synthetic steps are as follows:
[0047] Anhydrous acetone (70 g) was added to the three-neck reactor. After cooling the reactor to -15°C, 7.2027 g (0.068 mol) of BrCN was added and stirred while lowering the temperature below -25°C. Another 8.5684 grams (0.02 moles) of phosphorus compounds A and Et 3 N 6.1321 g (0.0606 mol) was thoroughly mixed and dissolved in anhydrous acetone (100 g), then slowly dropped into the reactor through a feeding funnel, and the temperature was maintained at -30° C., and the reaction was maintained for 2 hours. When the reaction temperature returned to -30°C, the reaction solution was dropped into deionized water for washing to remove ammonium bromide salt. After filtration, the resulting precipitate was dissolved in CH 2 Cl 2 / H 2 O for extraction. The organic phase...
Synthetic example 3
[0048] Synthesis Example 3: Synthesis of Compound A-ep
[0049] According to the embodiment of the present invention, A-ep is synthesized with phosphorus compound A. Its synthetic steps are as follows:
[0050] Take 214 grams of phosphorus compound A and 925 grams of epichlorohydrin into a 3-liter reactor, stir under normal pressure to form a uniform mixed solution, raise the reaction temperature to 70°C under 190mmHg absolute pressure and within 4 hours Add 200 g of 20% sodium hydroxide solution in batches, and add the water in the reactor azeotropically at the same time. After the reaction, the cyclochloropropane and the solvent were distilled clean by vacuum distillation, the product was dissolved in methyl ethyl ketone and deionized water, the sodium chloride in the resin was washed with water, and the solvent was distilled clean by vacuum distillation to obtain a white The epoxy equivalent of A-EPOXY (A-ep) is 242.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com