Method for production of antibody
A technology for antibody preparation and antibody, applied in the direction of antibodies, chemical instruments and methods, anti-animal/human immunoglobulin, etc., can solve the problems of time-consuming and laborious, high cost of preparing antibodies, relying on professionals, etc., and achieve the goal of improving operation efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] In this example, the S100A10 protein was used as an antigen example to illustrate the preparation method of antibody IgG1.
[0111] Extraction of spleen cells (splenocyte)
[0112] After identifying the mouse spleen with naked eyes, take it out and wash it. Two spleens were placed on a mesh, broken with a cell scraper, and cells were collected by centrifugation (1300 rpm×3 minutes, 4° C.). Next, after suspending the cells in 3 mL of ACK lysis buffer, 10 mL of PBS(-) was added. The cells were recovered by centrifugation (1300 rpm×3 minutes, 4°C), and suspended in 6 mL of RPMI1640(-). Pass through a cell strainer to remove insoluble fat etc.
[0113] in vitro immunization
[0114] The concentration of the S100A10 protein antigen was measured using a detection kit (Dc protein assay kit, manufactured by BioRad). At this time, BSA (manufactured by Sigma) was used as a standard. The known concentration of S100A10 protein antigen was dispensed according to the specif...
Embodiment 2
[0134] In this example, the extraction of the anti-S100A10 antibody gene and the steps of expressing the antibody in Escherichia coli will be described.
[0135] DNA extraction, preparation of single-chain antibody scFv plasmid
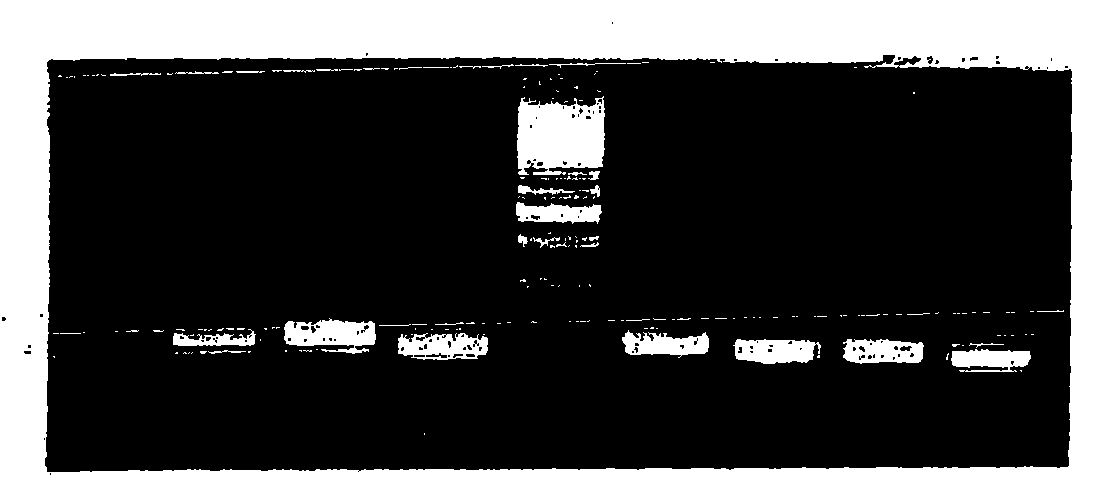
[0136] Use Isogen (manufactured by Nippongene) from 1.5×10 6 Total RNA was obtained from B cells or hybridomas after immunization of cells. Among them, immune cells obtained by the in vitro immunization step described in Example 1 above can also be used in the step.
[0137] Next, it was confirmed by 1.2% agarose gel (Agarose Gel) electrophoresis. The result obtained is as Figure 1A and Figure 1B shown.
[0138] Using the obtained total RNA as a template, single-stranded cDNA was synthesized using ReverTra Ace (manufactured by TOYOBO) reagent using mouse VH gene-specific primers or mouse VL gene-specific primers. Then, using the prepared cDNA as a template, use the primer-VH forward primer mixture / VH reverse primer mixture or VL forward prime...
Embodiment 3
[0152] The procedure described in Example 1 was repeated. However, in this example, S100A1 protein and S100A10 protein were used as antigens, and the same method as in Example 1 above was used to implement in vitro immunization, and the IgG1 positive rate was calculated. Figure 6 show the results thus obtained. From Figure 6 It can be seen that class switching to IgG1 occurs at a high probability of 60% or higher, although it may vary depending on the antigen.
[0153] In the in vitro (in vitro) immunization process of the present invention, LPS (40 μg / mL), IL-4, IL-5 (10ng / mL), anti-CD38 antibody, anti-CD40 antibody (1 μg / mL) are added once after antigen immunization. mL) and other stimuli, culturing for 4 days in the presence of stimuli can achieve effective class switching.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com