Kit for detecting H1N1 influenza A virus by real-time fluorescence RT-PCR
A RT-PCR, real-time fluorescence technology, used in fluorescence/phosphorescence, microorganism-based methods, and microbial determination/inspection to achieve good sensitivity, improve reliability and accuracy, and avoid false negatives and false positives.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the development of type A H1N1 influenza virus detection reagent
[0042] 1. Design of primers and probes: through sequence comparison and analysis of the nucleic acid sequences of the reported influenza A (H1N1) viruses, the H1 subtype HA gene and the N1 subtype NA gene of the influenza A (H1N1) virus were respectively used as amplification targets According to the basic principles of primer and probe design, multiple pairs of primers and probes are designed by software and manually.
[0043] 2. Selection of clinical samples: According to relevant domestic and foreign literature reports, throat swabs, nasopharyngeal secretions and other respiratory samples as well as serum samples can be used
[0044] 3. Establishment and optimization of the reaction system
[0045] Sample preparation: use the constructed plasmid containing the target amplification region as the positive quality control product for the detection of influenza A (H1N1) virus; Respiratory sy...
Embodiment 2
[0054] Embodiment 2: Influenza A (H1N1) virus detection kit and its use
[0055] 1. Prepare a kit including the following components: 1 tube of RNA extraction solution (50ml / tube), 1 tube of H1 subtype primer-probe mixture (50 μl / tube), 1 tube of N1 subtype primer-probe mixture (50 μl / tube) ) 1 tube, RT-PCR reaction solution (720μl / tube) 1 tube, RT-PCR enzyme system (150μl / tube) 1 tube, negative quality control (200μl / tube) 1 tube, H1 subtype positive quality control ( 100μl / tube) 1 tube, N1 subtype positive quality control (100μl / tube) 1 tube.
[0056] 2. Specimen collection, transportation and storage
[0057] Specimens will be collected by clinicians according to the actual situation. Testable specimens include throat swabs, nasopharyngeal secretions, and serum. The collection methods are as follows: ① nasopharyngeal secretions: natural expectoration or induced sputum suction (such as positive oxygen pressure method, disposable baby suction tube method, and nebulized stea...
Embodiment 3
[0072] Example 3: Application of Influenza A (H1N1) Influenza Virus Detection Kit to Detect Samples
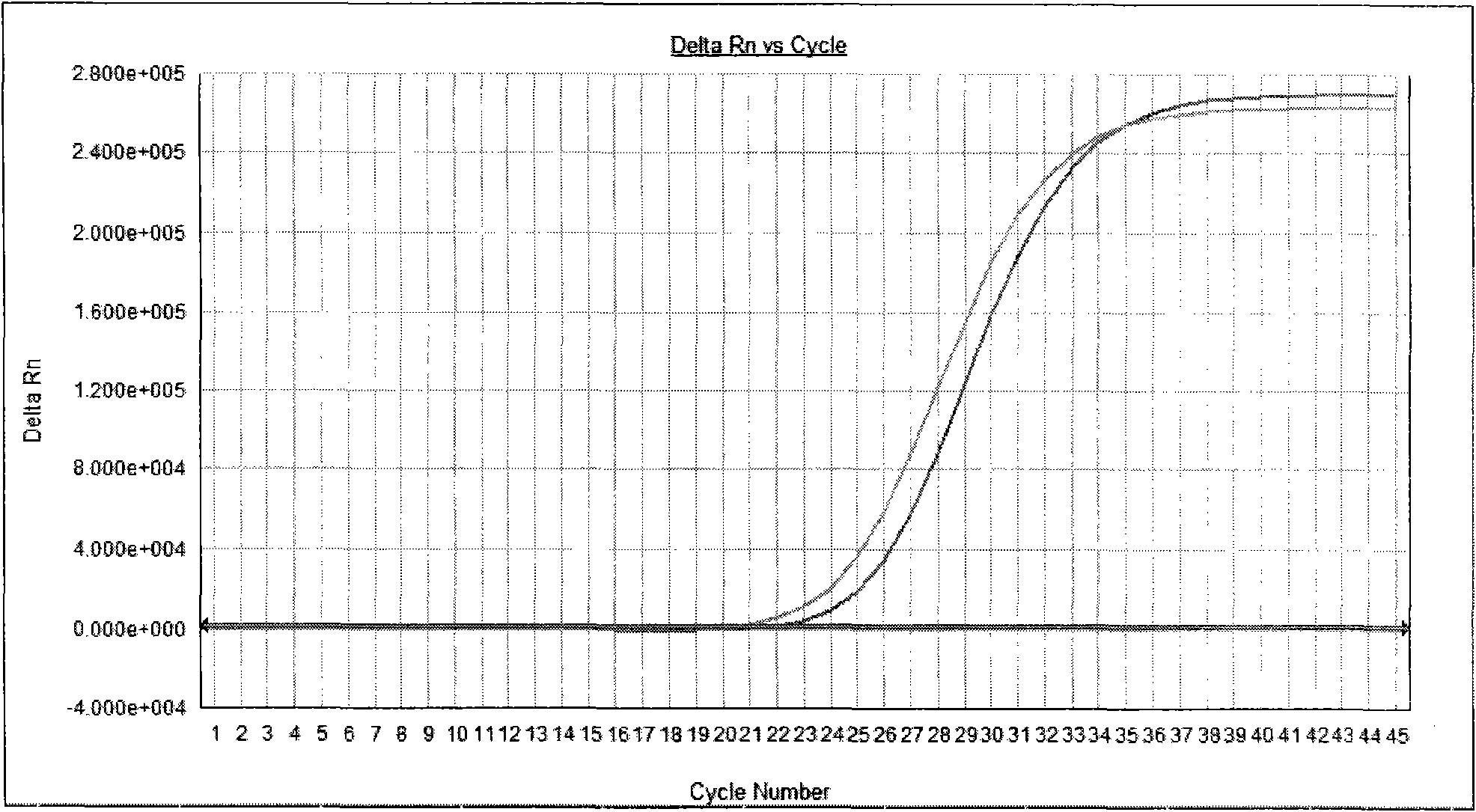
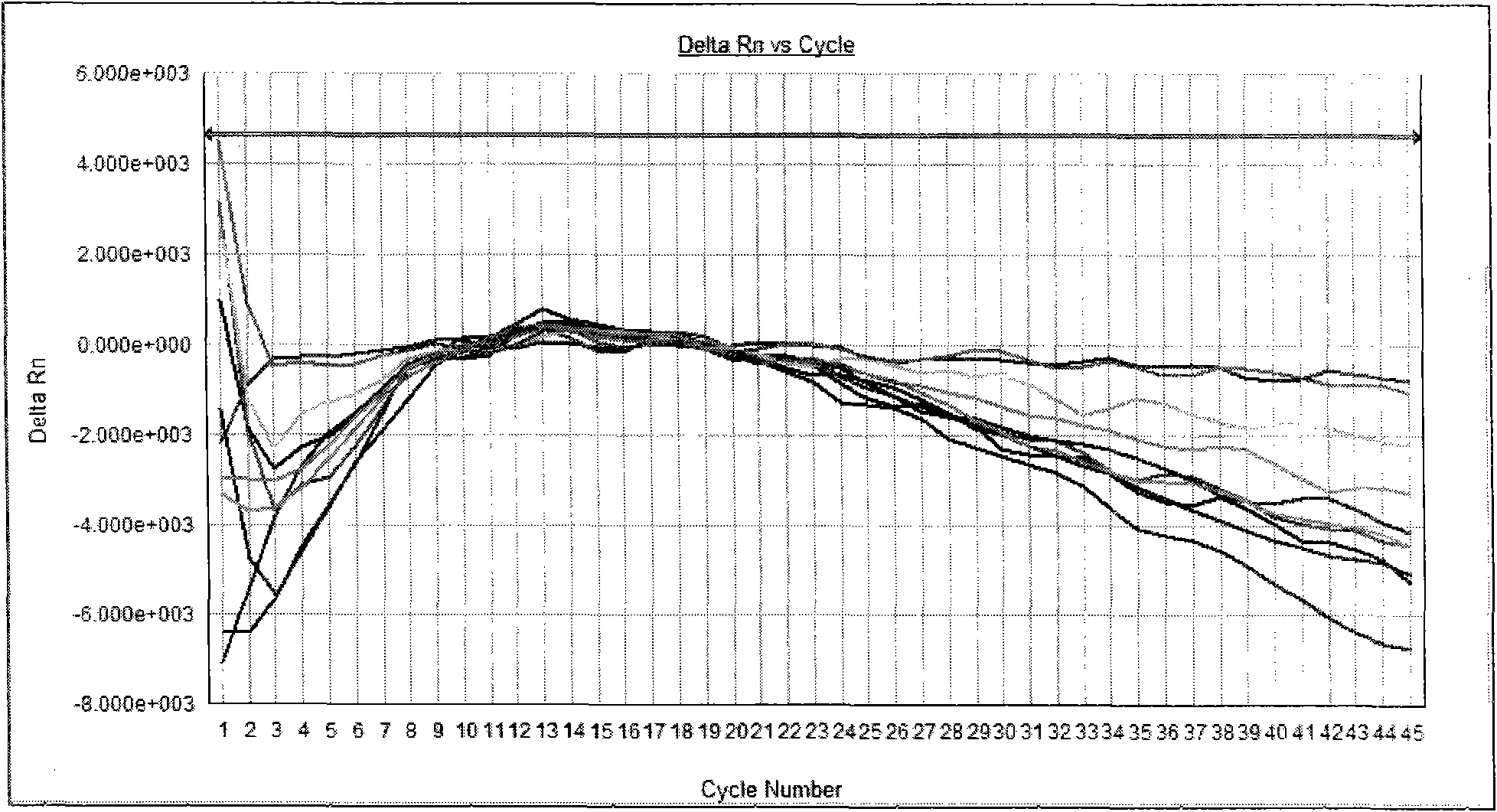
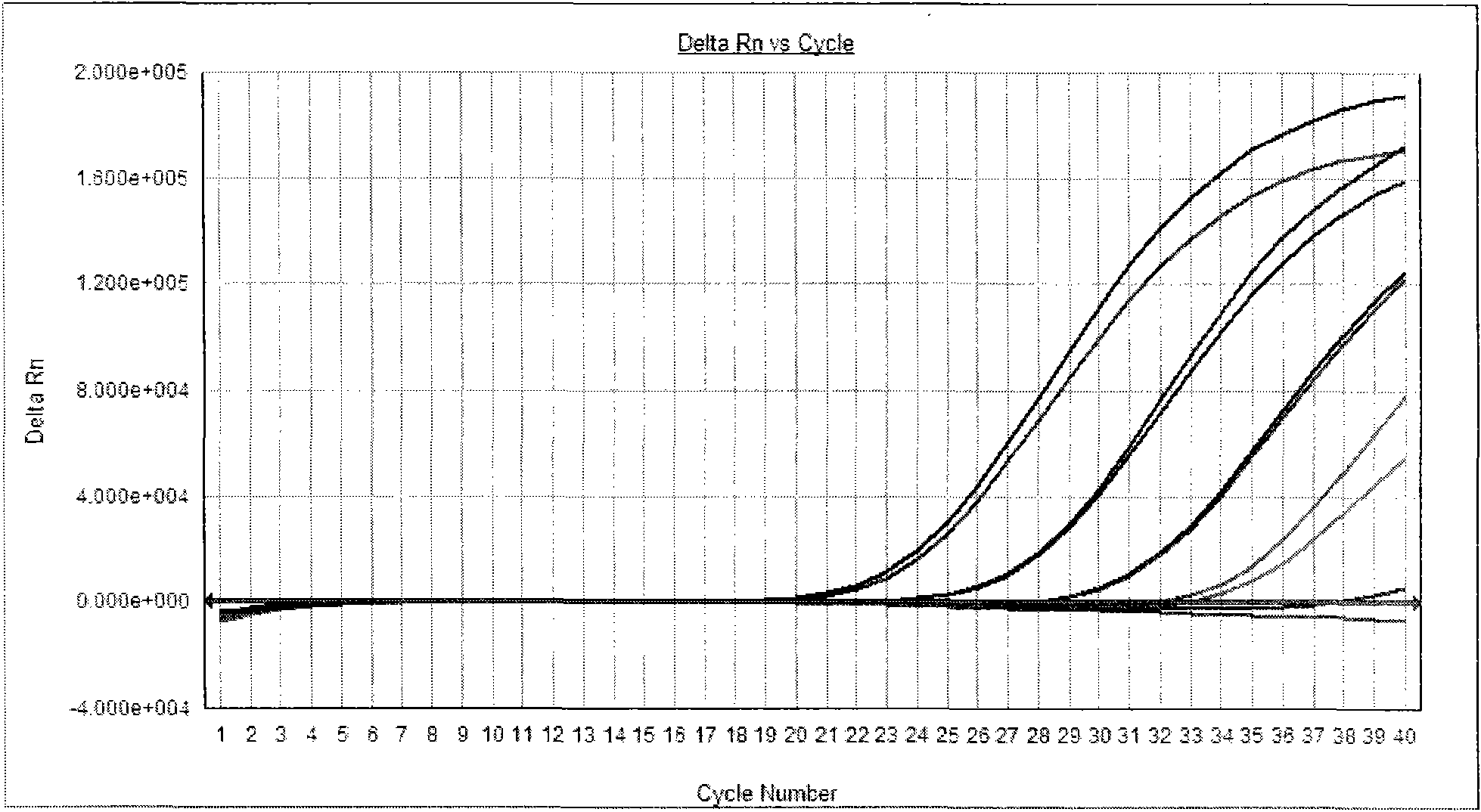
[0073] With positive quality control substance, negative quality control substance, specific reference substance in embodiment 1 as samples to be tested, detect these samples respectively according to the method of embodiment 2, positive quality control substance detection result is positive, negative quality control substance and The test results of specific reference products were all negative (see attached figure 1 ,2).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com