Reagent and method for detecting novel coronavirus
A new type of coronavirus technology, applied in the field of clinical medical nucleic acid molecular detection, can solve problems such as high biosafety risks, unstable quality, and destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
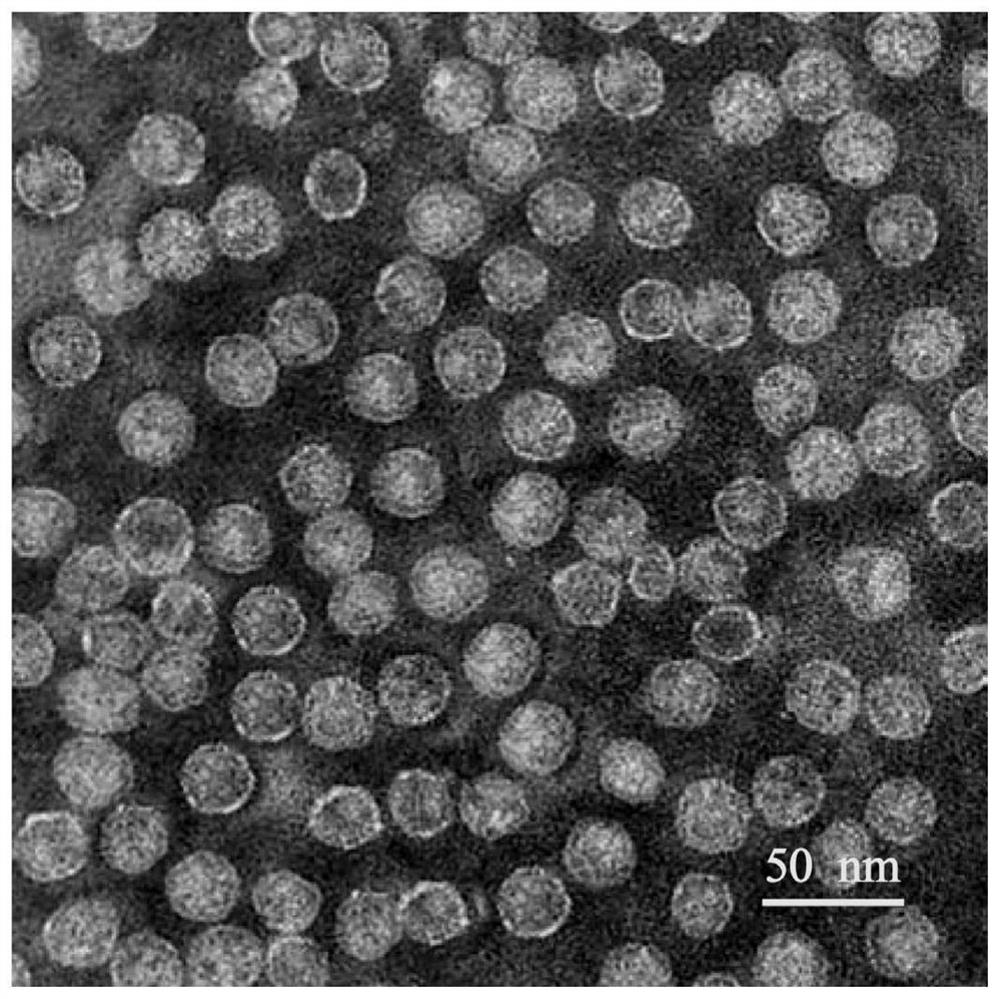
[0095] This paper also includes the preparation method of the above-mentioned pseudovirus, including introducing the nucleic acid construct containing the nucleic acid molecule described herein into the engineered cell, and cultivating the engineered cell under the conditions of transcription and / or translation of the nucleic acid construct, and optionally by said Engineered cells obtain and purify pseudoviral particles. Those skilled in the art are aware of common engineering cell culture methods, and methods for obtaining and purifying virus particles from engineered cells, such as sonication, organic solvent precipitation, and the like.
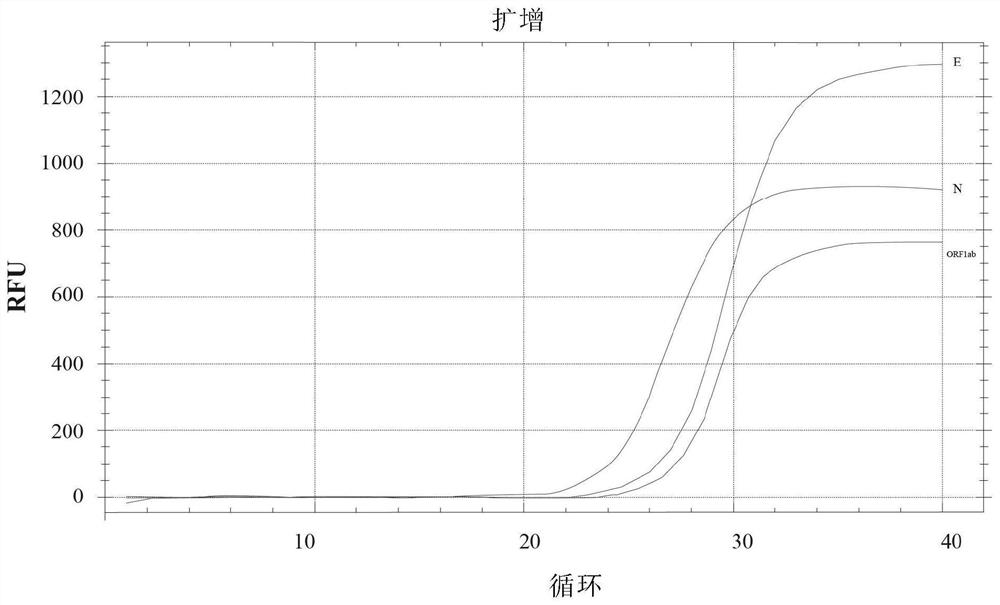
[0096]For example, in the embodiment of the novel coronavirus positive pseudovirion (positive control), the preparation process of the present invention includes: (1) obtaining ORF1ab gene, N gene and E gene sequence for novel coronavirus, such as sequence SEQ ID NO Shown in: 1; Described ORF1ab gene, N gene and E gene are obtained by synt...
Embodiment 1
[0149] Embodiment 1, the construction of pseudovirus quality control product expression vector
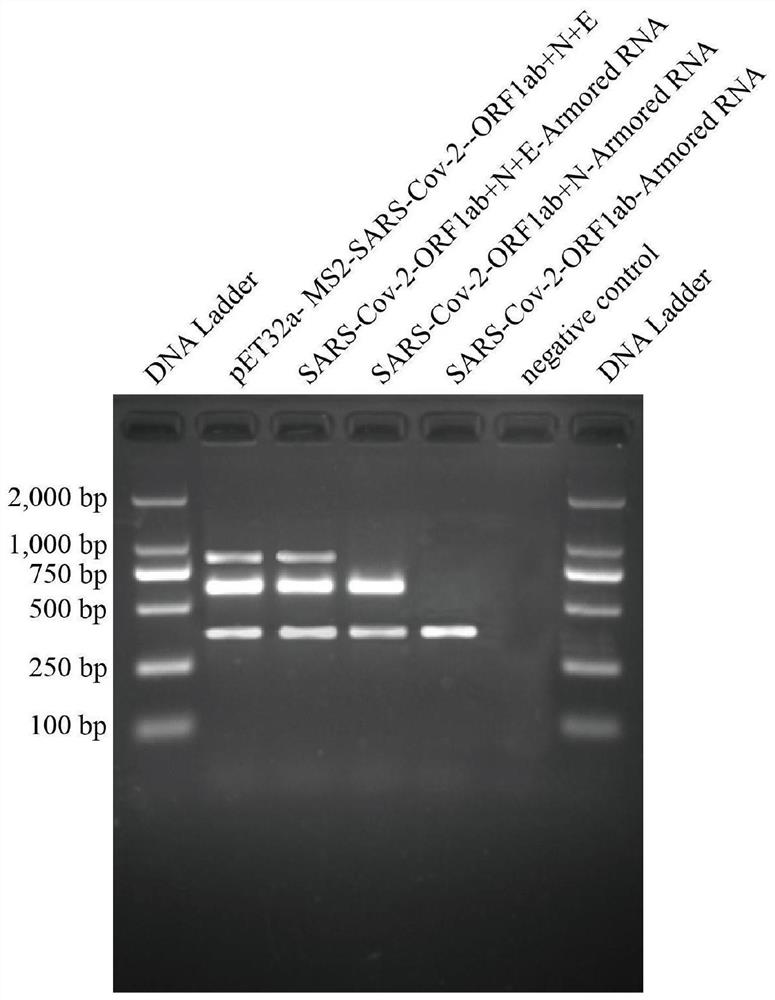
[0150] 1. The construction of the pseudovirus expression vector pET32a-MS2-SARS-COV2-ORF1ab+N+E of three genes (ORF1ab+N+E):
[0151] Refer to the MS2 phage genome sequence (Reference Sequence: NC_001417.2) in the NCBI database and the ORF1ab gene, N gene, and E gene of SARS-Cov-2 (Novel coronavirus nucleic acid detection published by the Chinese Center for Disease Control and Prevention of Viral Disease Prevention and Control) Primer and probe sequence coverage area), prepared by artificial synthesis, including the MS2 phage maturation enzyme coding gene, capsid protein coding gene, packaging site sequence, cDNA sequence corresponding to the new coronavirus nucleic acid fragment and auxiliary enzyme cutting site The auxiliary multiple cloning site is located in the middle of the ORF1ab, N and E gene fragments. The MS2 phage sequence is shown in SEQ ID NO: 2 in the sequence table,...
Embodiment 2
[0156] Embodiment 2, the preparation of pseudovirus quality control product
[0157] (1) Transform the plasmid expression vector prepared in Example 1 into Escherichia coli BL21 competent cells, spread it on a nutrient agar plate containing ampicillin (final concentration is 50 μg / ml), and culture it at 37°C for 18h (nutrient agar The specific preparation method of the plate is as follows: Tryptone 1g, NaCl 0.5g, yeast extract 0.5g, agar powder 1.5g, dissolved in 90mL ddH 2 O, adjust the pH to 7.0-7.2, then add ddH 2 O and dilute to 100mL, autoclaved for later use. Then pick positive monoclonal bacteria and inoculate them in LB liquid medium containing ampicillin for overnight culture.
[0158] (2) Inoculate 2ml of overnight cultured bacterial liquid into 200ml of ampicillin-resistant LB liquid medium (final concentration of ampicillin is 100μg / ml), shake at 200rpm until the OD value is about 0.6-0.8 (liquid LB medium The specific preparation method is: tryptone 1g, NaCl 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com