Surface-modified salbutamol suspension type non-Freon inhalation aerosol and preparation method thereof
A technology of albuterol and surface modification, which is applied in the directions of aerosol delivery, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
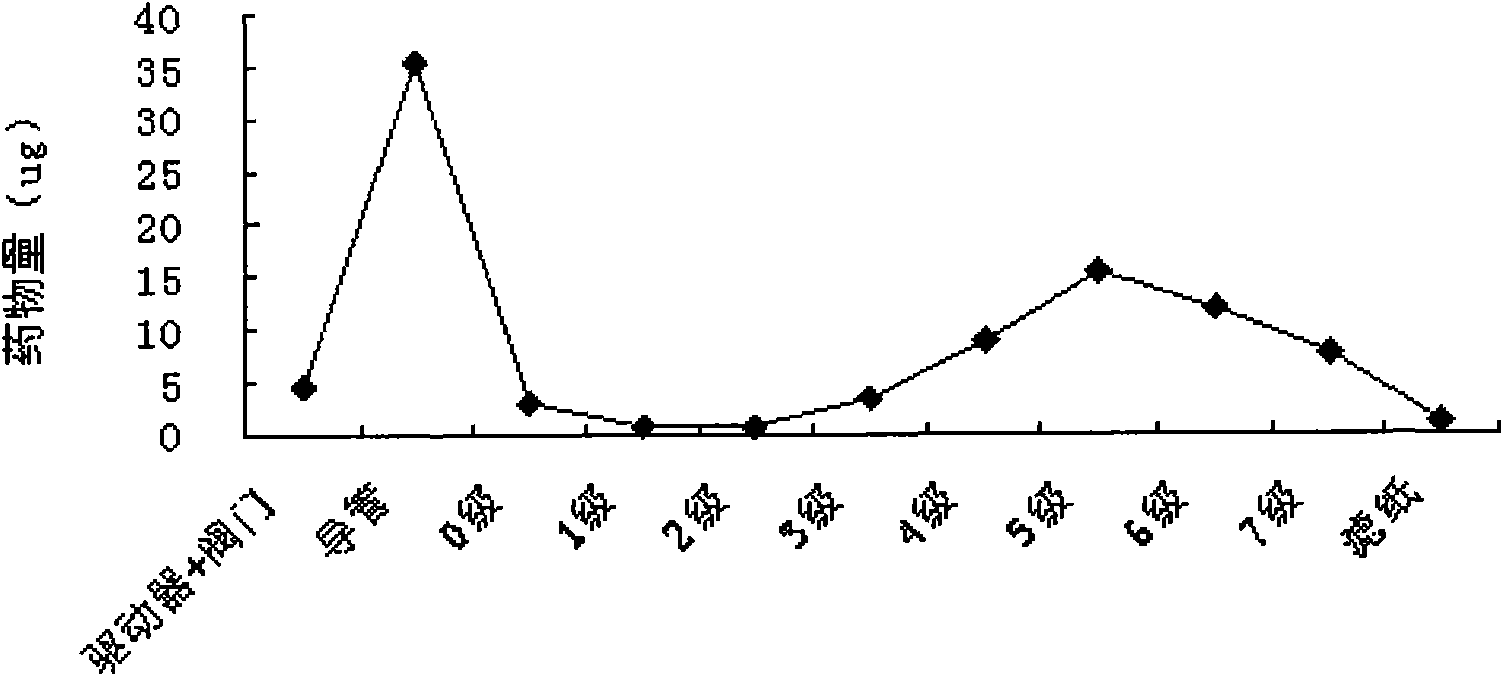
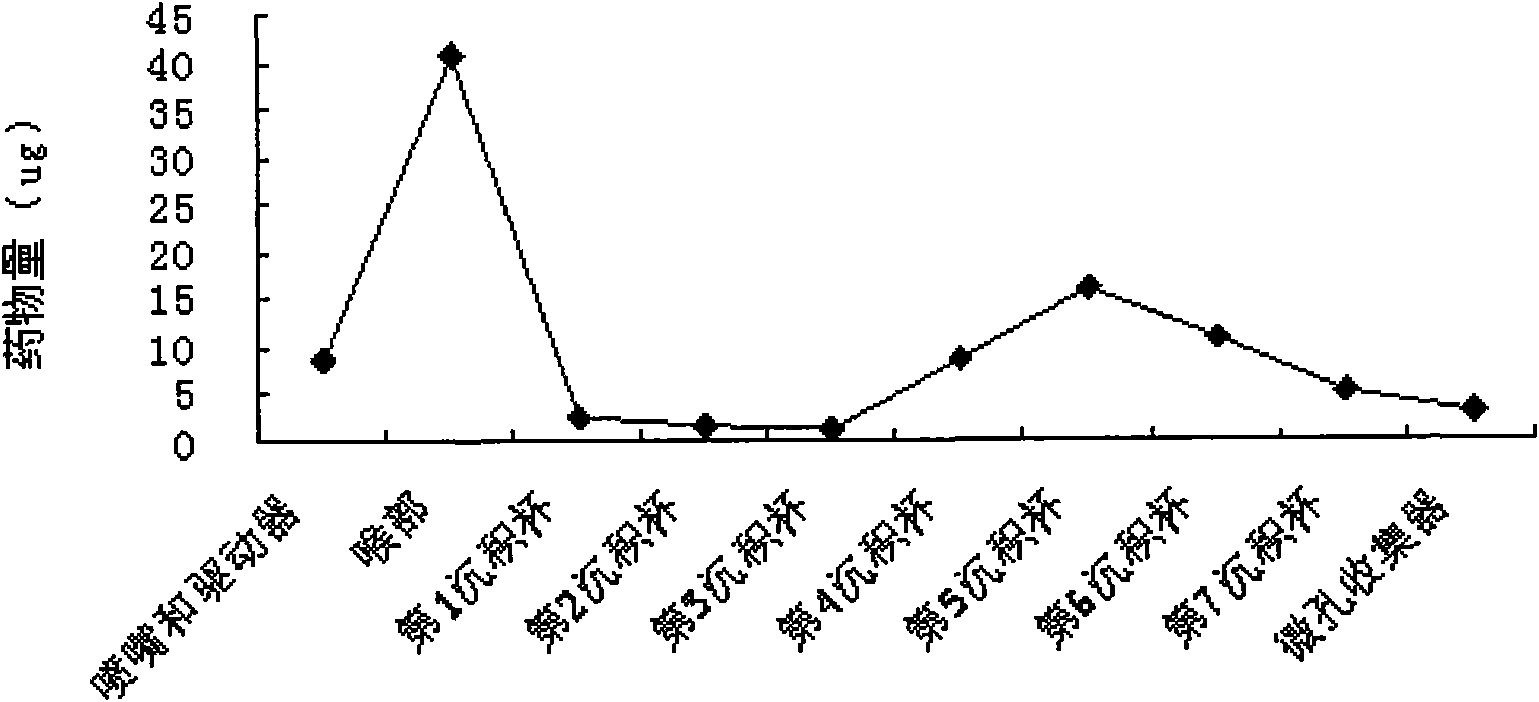
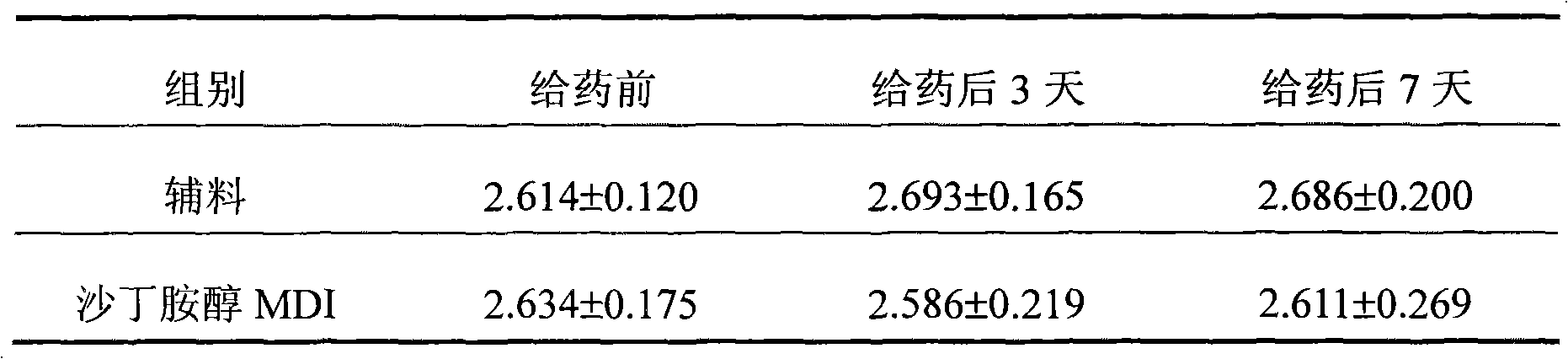
Image
Examples
Embodiment 1
[0060] Take 0.15mg of lecithin in 150g of absolute ethanol, dissolve it, add 100g of salbutamol sulfate with an average particle size of less than 10 microns, stir and disperse for 10 minutes, filter to remove the solvent and unadsorbed phospholipids, and dry at 50°C to obtain 100.0001g of average Particles with a particle size of 10 microns. Wherein, the weight of phospholipid is 0.0001% of the salbutamol sulfate weight.
Embodiment 2
[0062] Take 5g of soybean lecithin in 790g of absolute ethanol, after dissolving, add 100g of salbutamol sulfate with an average particle size of less than 10 microns, use a shearing machine to shear and disperse for 5 minutes, filter to remove the solvent and unadsorbed phospholipids, dry at 50°C, and air flow Pulverized to obtain 100.03 g of particles with an average particle diameter of 5 micrometers. Wherein, the weight of phospholipid is 0.03% of the salbutamol sulfate weight.
Embodiment 3
[0064] Get 0.4g of soybean lecithin in 400g of absolute ethanol, after dissolving, add 100g of levosalbutamol hydrochloride, ball mill for 20 minutes, the others are the same as in Example 2, and obtain 100.001g of particles with an average particle diameter of 2 microns. Wherein, the weight of phospholipid is 0.001% of the weight of levosalbutamol hydrochloride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com