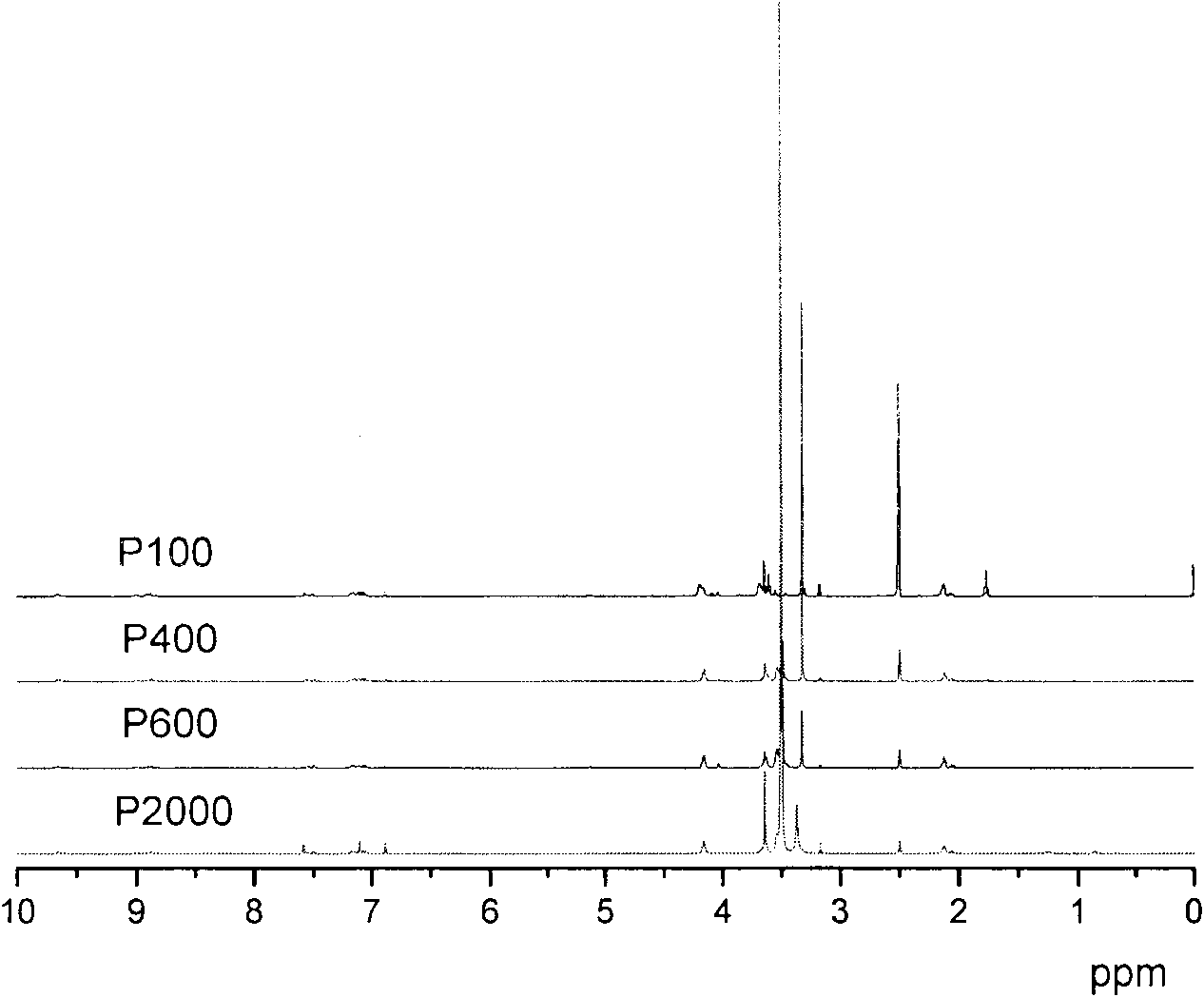
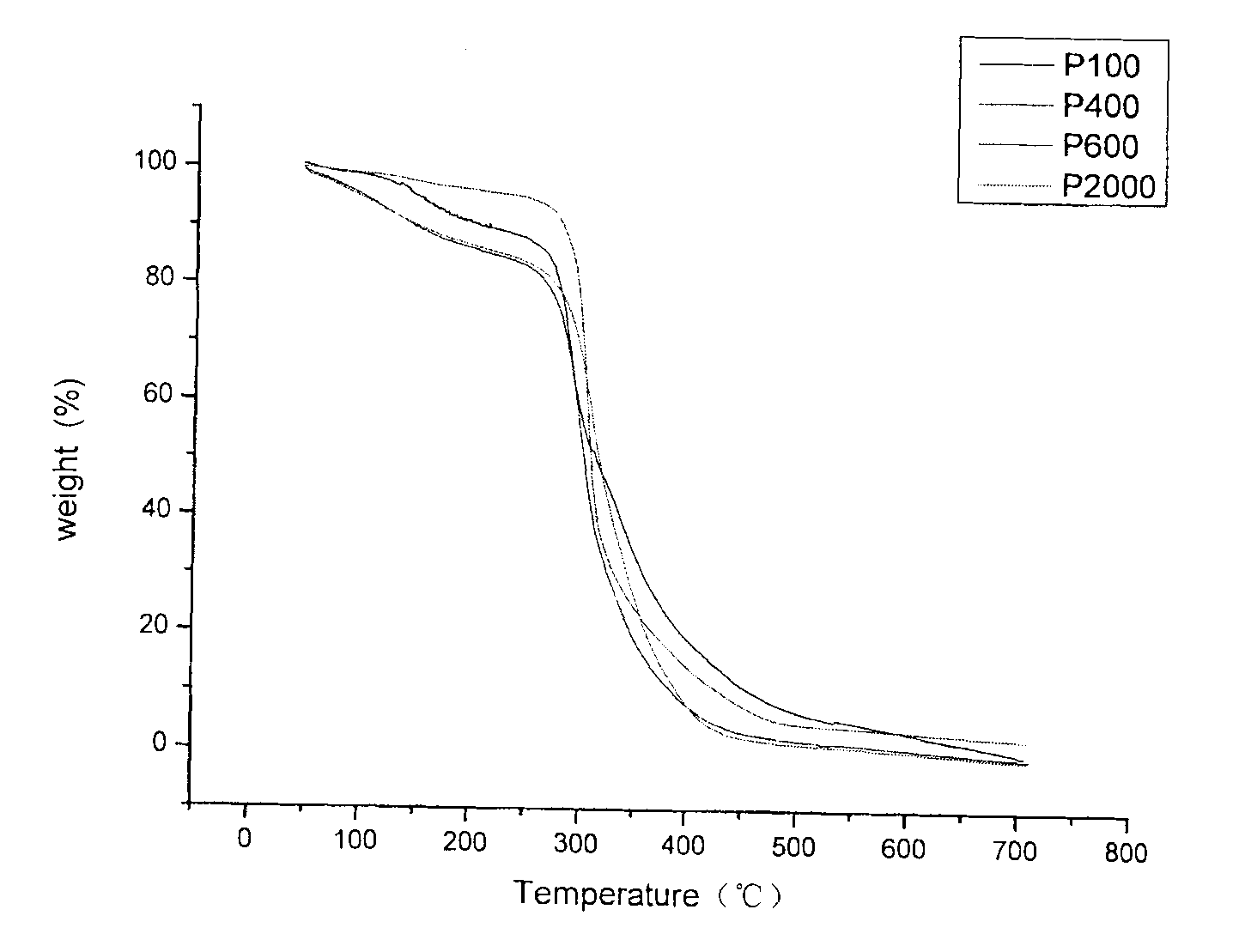
Imidazole salt type cationic polyurethane and preparation method thereof
A cationic polyurethane technology, which is applied in the field of imidazolium salt cationic polyurethane and its preparation, can solve problems not involving nitrogen heterocyclic compounds, and achieve good protection and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of imidazolate diol: Put 20g of dibromoneopentyl glycol in a 250mL single-necked flask, add 100mL of solvent THF and 27g of N-ethylimidazole in sequence, and reflux in an oil bath at 70°C for 10h. The solvent THF was removed by rotary evaporation to obtain ethyl imidazolium diol.
[0021] Preparation of imidazolium salt cationic polyurethane: under nitrogen protection, successively add 5.6g of polyethylene glycol with a weight-average molecular weight of 600, 30mL of solvent THF, and 2.6mL of toluene-2,6-diisocyanate into a 100mL three-necked flask, dropwise Two drops of catalyst dibutyltin dilaurate were pre-polymerized in an oil bath at 50°C for 2 hours, and then 5.0 g of the prepared ethylimidazolium salt diol was added to react for 22 hours. Methanol precipitation yields cationic polyurethane.
Embodiment 2
[0023] Preparation of imidazolate diol: Put 30g of dibromoneopentyl glycol in a 250mL one-necked flask, add 100mL of solvent THF and 18mL of N-methylimidazole in sequence, and put it in an oil bath at 70°C for reflux reaction for 10h. The solvent THF was removed by rotary evaporation to obtain methylimidazolium diol.
[0024] Preparation of cationic polyurethane: under nitrogen protection, 3.2g of methylimidazolium diol, 12.7g of polyethylene glycol with a weight average molecular weight of 106, 30mL of solvent THF, and 1.8mL of toluene-2,4-diisocyanate were added to the In a 100mL three-necked flask, two drops of catalyst dibutyltin dilaurate were added dropwise, and placed in an oil bath at 40°C for 24 hours of reaction. Methanol precipitation yields cationic polyurethane.
Embodiment 3
[0026] Preparation of imidazolate diol: Put 15g of dibromoneopentyl glycol in a 250mL single-necked flask, add 100mL of solvent THF and 9mL of N-methylimidazole in sequence, and place it in an oil bath at 70°C for reflux reaction for 10h. The solvent THF was removed by rotary evaporation to obtain methylimidazolium diol.
[0027] Preparation of cationic polyurethane: under nitrogen protection, add 1.4g of methylimidazolium salt diol, 1.0g of double-terminated hydroxyl polycaprolactone with a molecular weight of 1400, 30mL of solvent THF, and 6.3mL of hexyl diisocyanate into a 100mL three-port In the flask, add two drops of catalyst dibutyltin maleate dropwise, and place it in an oil bath at 50°C for 24 hours. Methanol precipitation yields cationic polyurethane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com