A kind of preparation method containing imidazolium cationic antistatic polyurethane
A technology containing imidazolium cation and ionic type, applied in the field of polymer material preparation, can solve problems such as affecting product quality, disasters, affecting product appearance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
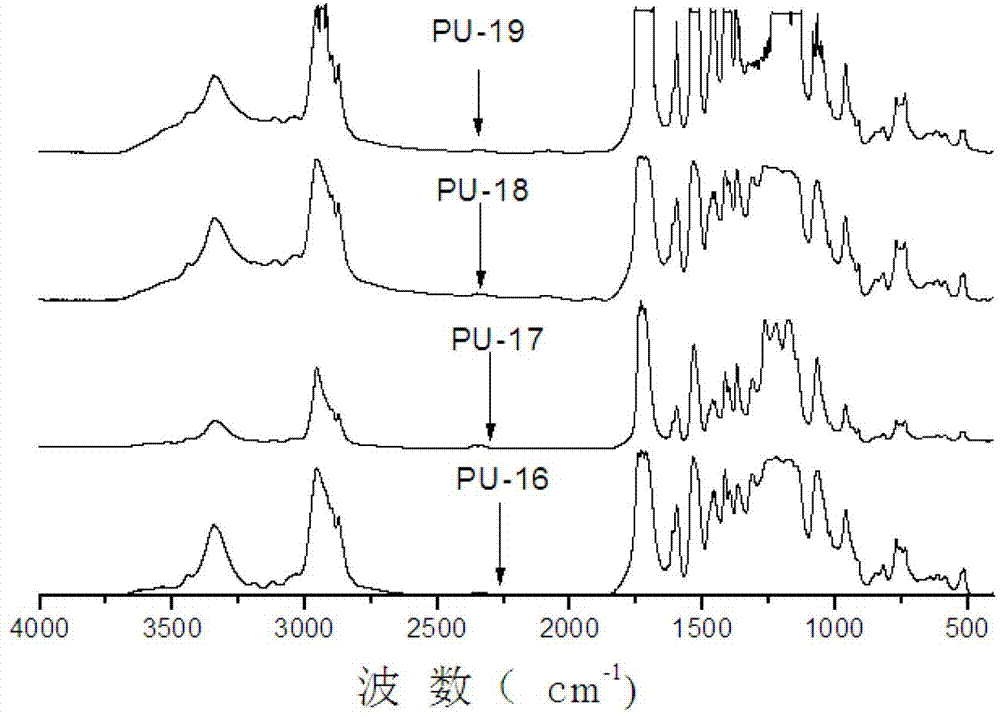
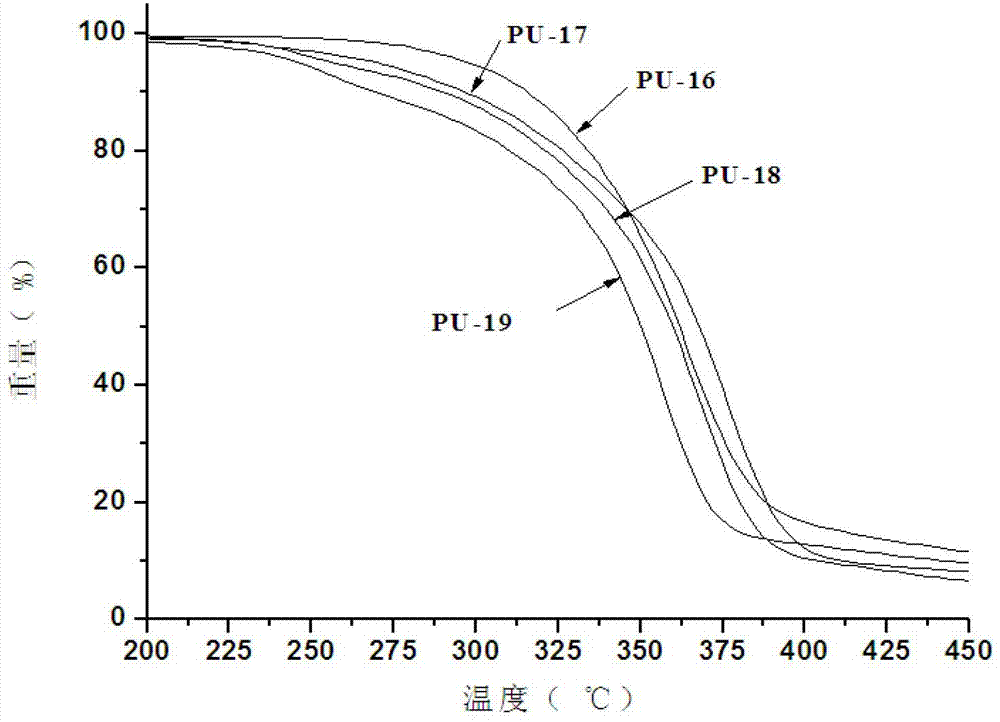
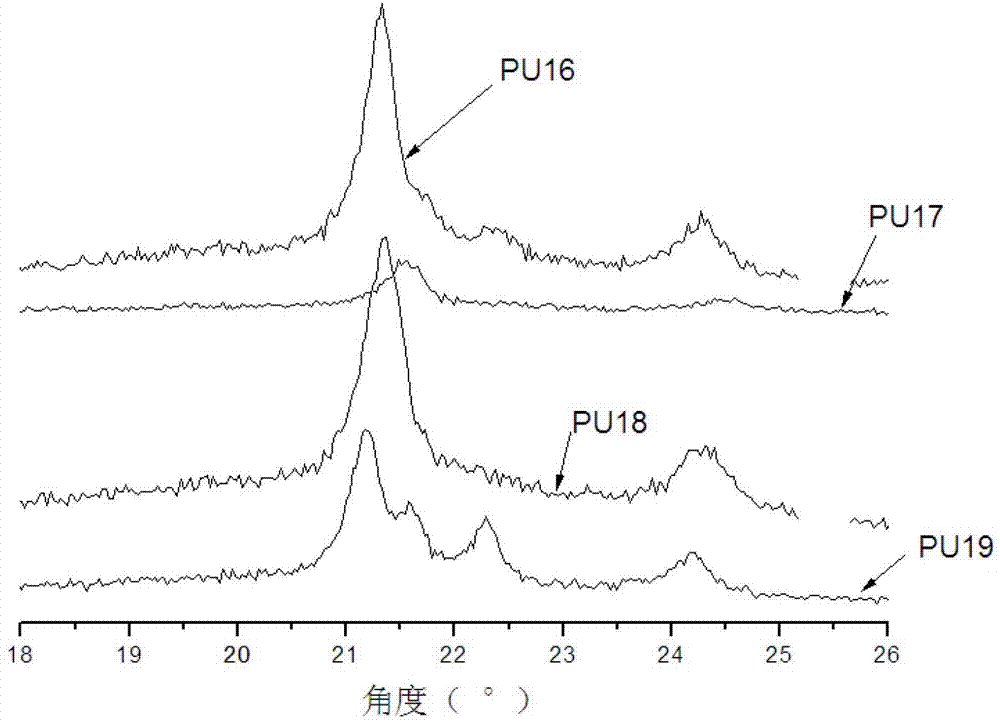
Image
Examples
Embodiment 1
[0036] Take 30 g of imidazole (441 mmol) in a flask, dissolve imidazole with 882 mmol of methanol, add 37.96 g (441 mmol) of methyl acrylate after the imidazole is completely dissolved, stir the reaction at 0 °C for 72 h, and distill off the methanol under reduced pressure solvent and excess methyl acrylate to obtain imidazole methylated derivatives.
[0037] The obtained methylated imidazole and 54.68 g (441 mmol) of 2-bromoethanol were dissolved in 882 mmol of acetone, reacted at a temperature of 60 °C for 1 h, and the acetone solvent and excess 2-bromoethanol were distilled off under reduced pressure to obtain the terminal hydroxymethyl Esterified imidazolium ionic liquid. Take 11.67 g (50 mmol) of hydroxy-terminated methylated imidazolium ionic liquid and 50 g (25 mmol) of polyethylene glycol with a molecular weight of 1000 at a temperature of 200 ℃ for 1 h to obtain a dihydroxy-terminated imidazolium cationic antistatic polyether glycol.
[0038]Take 1 mol of the obtain...
Embodiment 2
[0040] Take 47 g (691 mmol) of imidazole in a flask, dissolve the imidazole with 41 mol of methanol, add 442 g (3455 mmol) of butyl acrylate after the imidazole is completely dissolved, stir the reaction at 70 °C for 1 h, and distill off the methanol under reduced pressure Solvent and excess butyl acrylate to obtain imidazobutin esterified derivatives.
[0041] Dissolve the obtained butylated imidazole and 428.48 g (3455 mmol) 2-bromoethanol in 41 mol acetone, react at a temperature of 0 °C for 72 h, and distill off the acetone solvent and excess 2-bromoethanol under reduced pressure to obtain terminal hydroxybutyrate Esterified imidazolium ionic liquid. Take 25 g (90 mmol) of hydroxy-terminated butyl esterified imidazolium ionic liquid and 90 g (45 mmol) of double-terminated hydroxyl polycaprolactone with a molecular weight of 4000, and stir and react at a temperature of 80 °C for 42 h to obtain a dihydroxy-terminated imidazolium cationic Antistatic polyester diol.
[0042]...
Embodiment 3
[0044] Take 20 g of imidazole (294 mmol) in a flask, dissolve the imidazole with 1.2 mol of methanol, add 45.1 g (352 mmol) of butyl acrylate after the imidazole is completely dissolved, stir the reaction at 45 °C for 56 h, and distill off the methanol under reduced pressure Solvent and excess butyl acrylate to obtain imidazobutin esterified derivatives.
[0045] Dissolve the obtained butylated imidazole and 44.7 g (361 mmol) 2-bromoethanol in 1.31 mol acetone, react at a temperature of 30°C for 40 h, and distill off the acetone solvent and excess 2-bromoethanol to obtain hydroxy-terminated butyl ester imidazolium ionic liquid. 35 g (127 mmol) of hydroxy-terminated butyl esterified imidazolium ionic liquid and 63.5 g (63.5 mmol) of double-terminated hydroxyl polycaprolactone with a molecular weight of 1000 were stirred and reacted at 160 °C for 10 h to obtain a dihydroxy-terminated imidazolium cationic antibiotic Electrostatic polyester diol.
[0046] Get 3 mol of gained pol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com