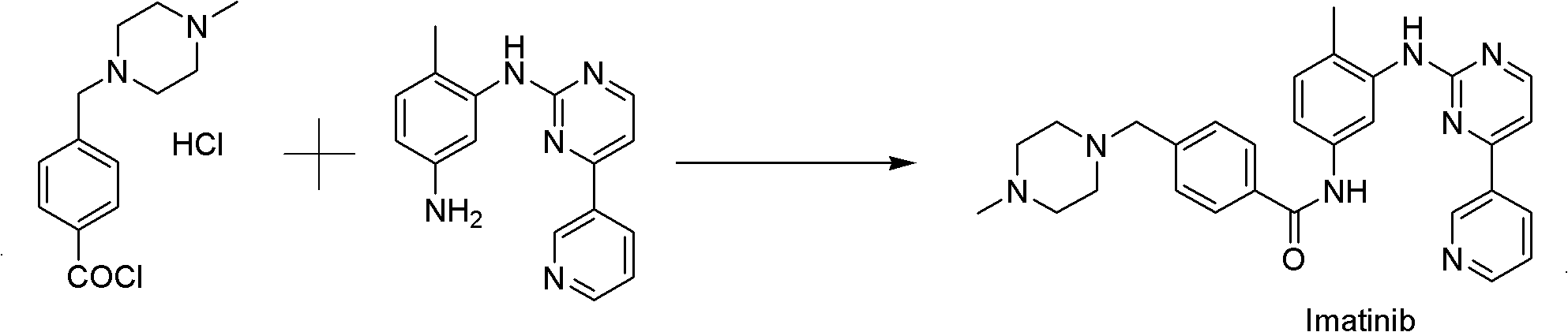
Preparation method of high-purity imatinib
A high-purity, pyridine technology, applied in the field of medicine, can solve the problems of a large amount of pyridine solvent, no recovery, large pollution, etc., and achieve the effects of simple operation process, mild reaction conditions, and reduced production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Install a mechanical stirrer and a thermometer on a 250mL four-necked bottle, and then add 1.77g (6.4mmol) of N-(5-amino-2-methylphenyl)-4-(3-pyridine)-2-pyrimidinamine and 50ml of pyridine, cooled to 5-10°C in an ice-water bath with stirring, and then added 3.0g (12.8mmol) of 4-[(4-methyl-1-piperazine)methyl]benzoyl chloride in batches After the addition of dihydrochloride, the ice-water bath was removed, and the temperature was raised to room temperature. After reacting for 2-3 hours, the reaction was stopped when the reaction was detected by HPLC, and the pyridine was evaporated under reduced pressure to recover it. Add 18ml of water to the residue and stir to dissolve the residue to form a transparent brown liquid, then extract twice with an appropriate amount of ethyl acetate, separate the organic phase and the water phase, and then use the water phase in an ice-water bath Adjust the pH to 9-10 with 25% ammonia water, then add 20ml of methanol, remove the ice-water...
Embodiment 2
[0017] Install a mechanical stirrer and a thermometer on a 1000mL four-necked bottle, then add 10g (36.1mmol) N-(5-amino-2-methylphenyl)-4-(3-pyridine)-2-pyrimidinamine and 250ml of pyridine, cooled to 5-10°C in an ice-water bath with stirring, then added 23.5g (72.12mmol) of 4-[(4-methyl-1-piperazine)methyl]benzoyl chloride di After the addition of hydrochloride, the ice-water bath was removed, and the temperature was raised to room temperature. After reacting for 2-3 hours, the reaction was stopped when the reaction was detected by HPLC, and the pyridine was evaporated under reduced pressure to recover it. Add 100ml of water to the residue and stir to dissolve the residue to form a transparent brown liquid, then extract twice with an appropriate amount of ethyl acetate, separate the organic phase and the water phase, and then use the water phase in an ice-water bath Adjust the pH to 9-10 with 25% ammonia water, then add 110ml of methanol, remove the ice-water bath, naturally...
Embodiment 3
[0019] Install a mechanical stirrer and a thermometer on a 1000mL four-necked flask, blow nitrogen into it, and then add 10g (36.1mmol) of N-(5-amino-2-methylphenyl)-4-(3-pyridine)-2 -Pyrimidinamine, 250ml acetonitrile and 23.7g (234.65mmol) triethylamine, placed in an ice-water bath to cool to 5-10°C under stirring conditions, then added 23.5g (72.12mmol) 4-[(4-methyl Base-1-piperazine) methyl] benzoyl chloride dihydrochloride. After the addition, the ice-water bath was removed, and the temperature was raised to room temperature. After reacting for 2-3 hours, the reaction was stopped when the HPLC detection reaction was complete, and evaporated under reduced pressure. Remove acetonitrile to recover it. Add 100ml of water to the residue and stir to dissolve the residue to form a transparent brown liquid, then extract twice with ethyl acetate, 75ml each time, separate the organic phase and the aqueous phase, then place the aqueous phase on ice Use 25% ammonia water in the wate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com