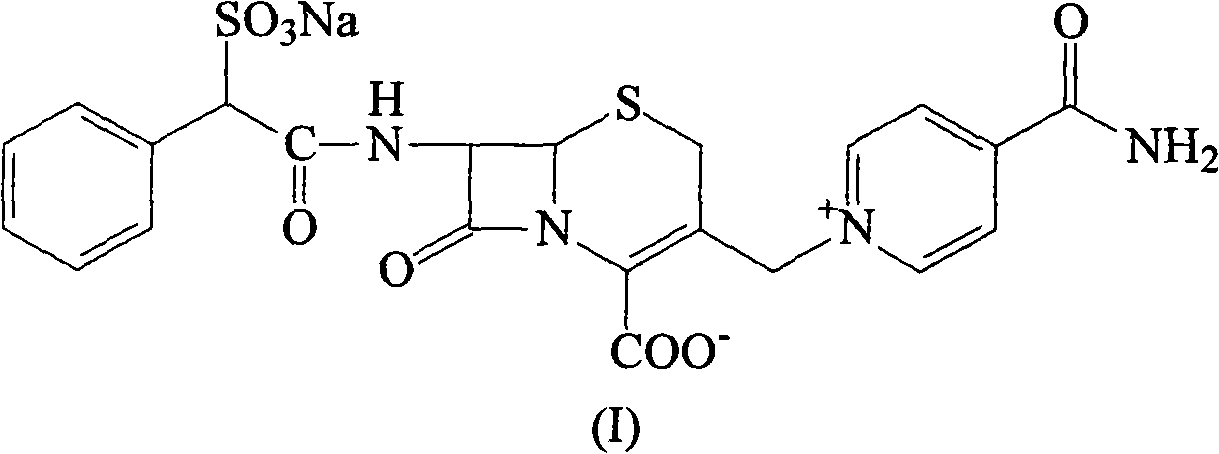
Cefsulodin sodium liposome injection
A technology of cefsulodin sodium and sulfsulodin sodium lipid, applied in the field of medicine, can solve problems such as leakage of encapsulated drugs, easy aggregation and fusion of liposomes, and achieve enhanced stability, excellent stability, and wide storage conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
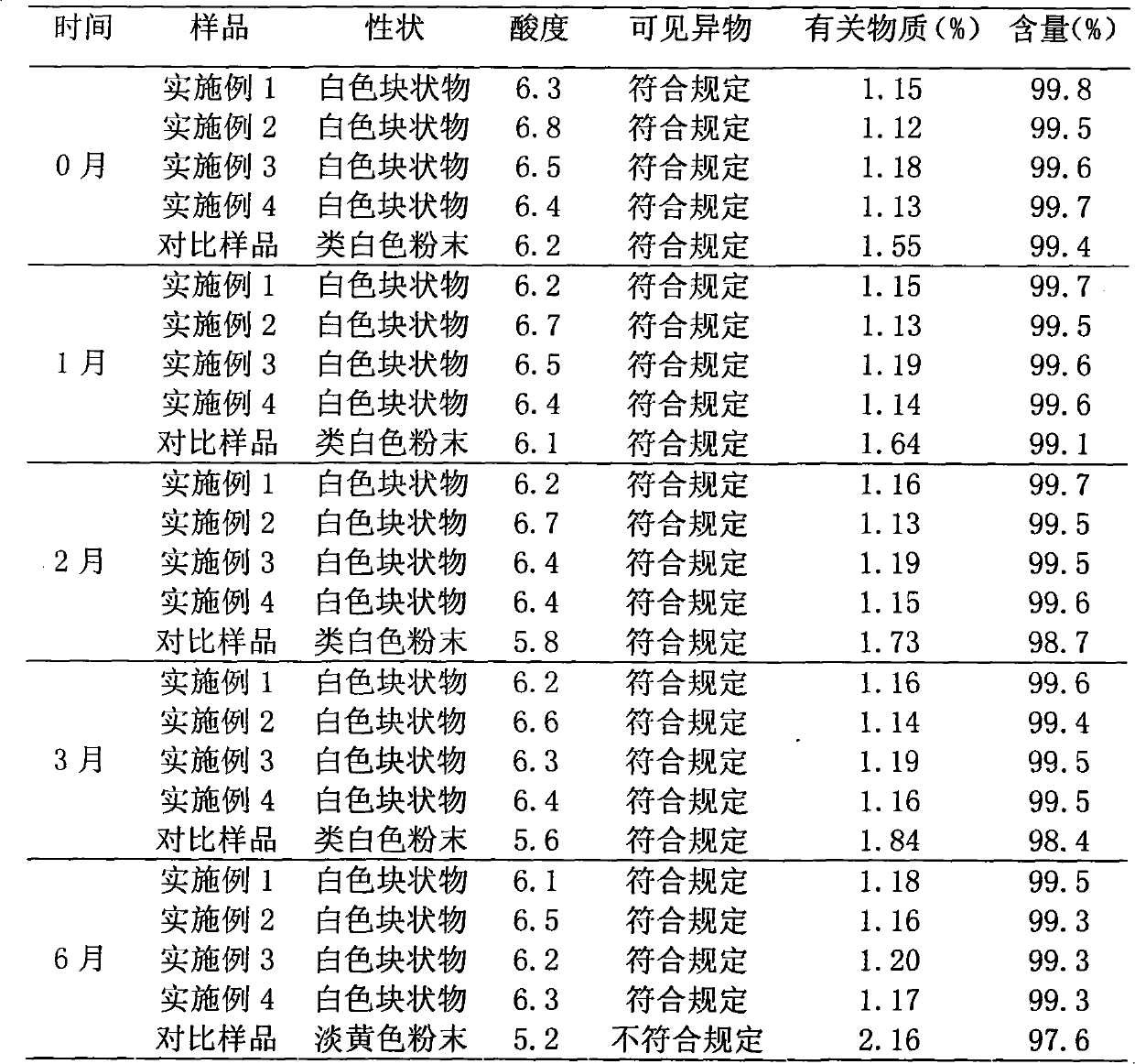
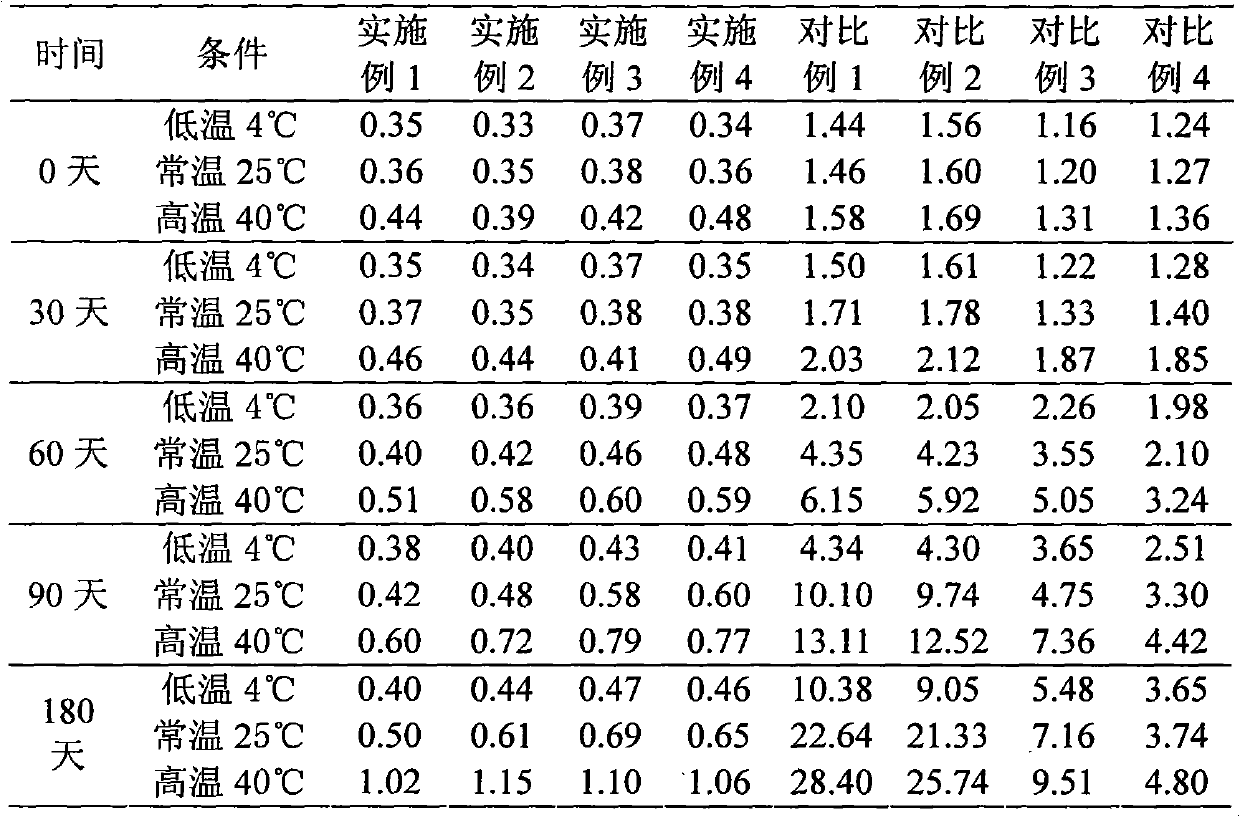
[0052] The preparation of embodiment 1 cefsulodin sodium liposome injection
[0053] Prescription (100 bottles):
[0054] Cefsulodin sodium 10g
[0056] Sodium Glycocholate 27g
[0057] Tween 8016g
[0059] Glucose 20g
[0060] Mannitol 60g
[0061] Preparation Process
[0062] (1) Add 50g egg yolk lecithin, 27g sodium glycocholate, and 16g Tween 80 into 800ml of a mixed solvent of dichloromethane and acetone at a volume ratio of 4:1, heat and stir to disperse evenly, and place on a rotary thin film evaporator Remove the organic solvent under reduced pressure to obtain a phospholipid film;
[0063] (2) Add 400ml of acetic acid-sodium acetate buffer solution with a pH value of 5.6, shake, stir to make the phospholipid film fully hydrated, high-speed homogeneous emulsification, and microporous membrane filtration to obtain a blank liposome suspension;
[0064] (3) Dissolve 10 g of cefsulodin sodium in 200 ml of...
Embodiment 1
[0066] The preparation of comparative example 1 cefsulodin sodium liposome injection
[0067] Prescription (100 bottles):
[0068] Cefsulodin sodium 10g
[0069] Egg yolk lecithin 50g
[0070] Tween 8016g
[0072] Glucose 20g
[0073] Mannitol 60g
[0074] The preparation process was the same as in Example 1, and cefsulodin sodium liposome injection without sodium glycocholate was prepared.
Embodiment 2
[0075] The preparation of embodiment 2 cefsulodin sodium liposome injection
[0076] Prescription (100 bottles):
[0077] Cefsulodin sodium 20g
[0078] Egg yolk lecithin 44g
[0079] Sodium Glycocholate 12g
[0080] Tween 806g
[0081] Sodium sulfite 1.2g
[0082] Glucose 17.5g
[0083] Mannitol 52.5g
[0084] Preparation Process
[0085] (1) Add 44g egg yolk lecithin, 12g sodium glycocholate, and 6g Tween 80 into 800ml of a mixed solvent of dichloromethane and acetone at a volume ratio of 4:1, heat and stir to disperse evenly, and place on a rotary film evaporator Remove the organic solvent under reduced pressure to obtain a phospholipid film;
[0086] (2) Add 500ml of citric acid-sodium citrate buffer solution with a pH value of 5.6, shake and stir to make the phospholipid membrane completely hydrated, high-speed homogeneously emulsified, and filtered through a microporous membrane to prepare a blank liposome mixture suspension;
[0087] (3) Dissolve 20 g of cefsu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com