Oxidizer for firework explosive agent and preparation thereof
A technology of fireworks and firecrackers and crackers, which is applied in the direction of explosives, etc., can solve the problems of reduced fire contact ability, lower firing rate, and high cost, and achieve the goals of reducing the decomposition temperature, increasing the oxygen release rate, and improving the quality of firecrackers Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
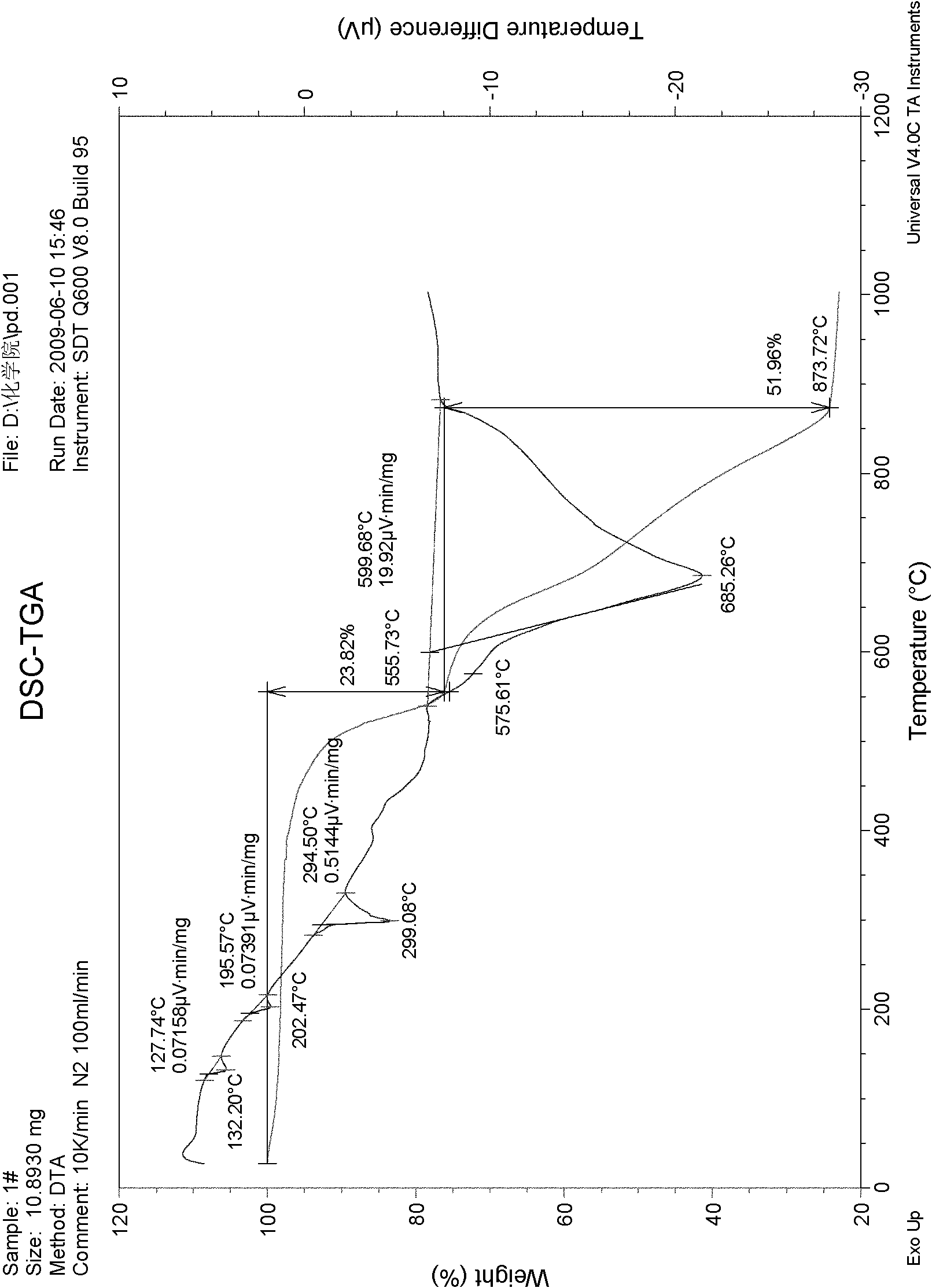
[0035] 40% of potassium perchlorate, 30% of potassium nitrate, and 30% of barium nitrate are used to form a mixture, then tartaric acid accounting for 3% of the total mass of the mixture is added to obtain the oxidant of the present invention, and finally mixed with combustible agent 3: 2 to form firecracker medicament to produce firecrackers. After testing, it was found that:
[0036] The explosion rate is over 95%;
[0037] The firing rate reaches over 98%;
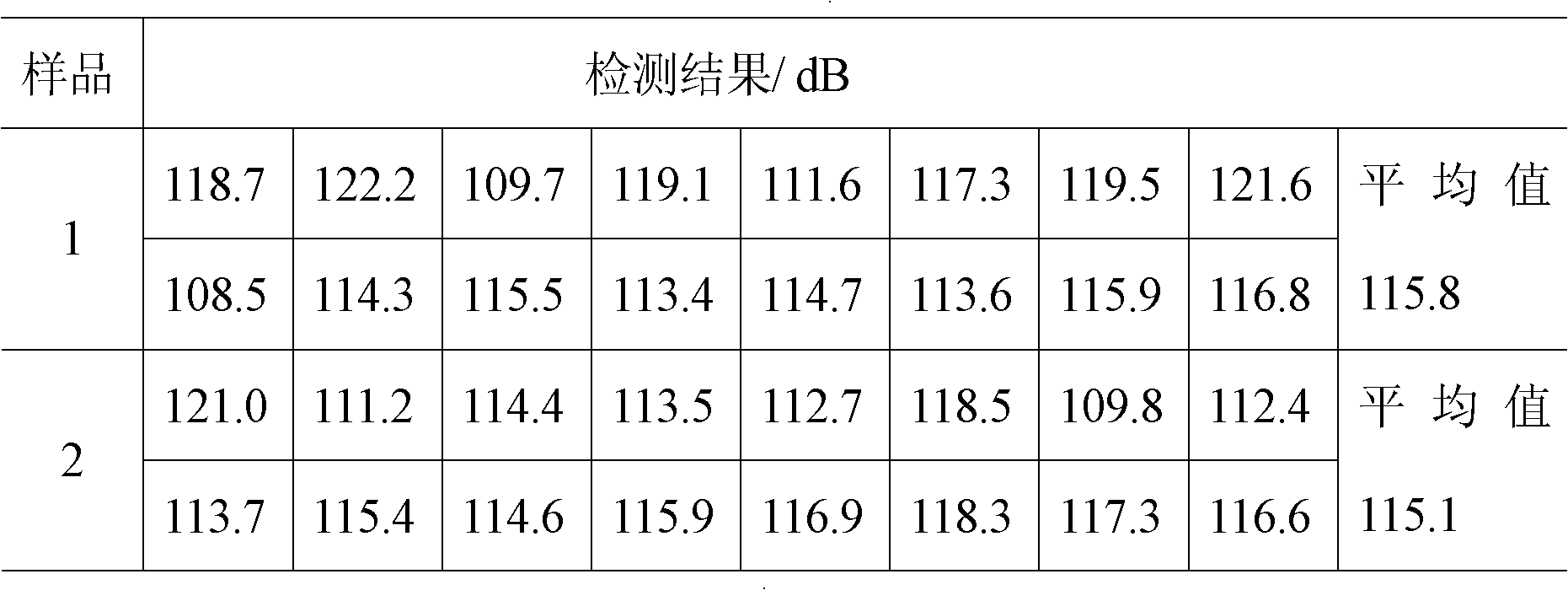
[0038] Sound level: 108.5dB ≤ sound level value should be ≤ 122.2dB;
[0039] The time for the gas product pressure to rise from 0.69MP to 2.07MP is 1.74ms,
[0040] The pH value of the pyrotechnic powder is in the range of 5 to 9;
[0041] Moisture content of pyrotechnic powder ≤ 1.5%;
[0042] The moisture absorption rate of pyrotechnic powder is ≤2.0%;
[0043] The thermal stability of the pyrotechnic powder is that there is no decomposition phenomenon at 75°C±2°C for 48 hours;
[0044] The friction sensitivity...
Embodiment 2
[0055] First, 20% of potassium perchlorate, 30% of potassium nitrate and 50% of barium nitrate are used to form a mixture, then tartaric acid accounting for 1% of the total mass of the mixture is added to obtain the oxidant of the present invention, and finally mixed with combustible agent 1:1 to form a firecracker agent to produce firecrackers. After testing, it was found that:
[0056] The explosion rate is over 95%;
[0057] The firing rate reaches over 98%;
[0058] Sound level: 105.5dB ≤ sound level value should be ≤ 120.2dB;
[0059] The time for the gas product pressure to rise from 0.69MP to 2.07MP is 1.75ms;
[0060] The pH value of the pyrotechnic powder is in the range of 5 to 9;
[0061] Moisture content of pyrotechnic powder ≤1.02%;
[0062] The moisture absorption rate of pyrotechnic powder is ≤2.0%;
[0063] The thermal stability of the pyrotechnic powder is that there is no decomposition phenomenon at 75°C±2°C for 48 hours;
[0064] The friction sensitivi...
Embodiment 3
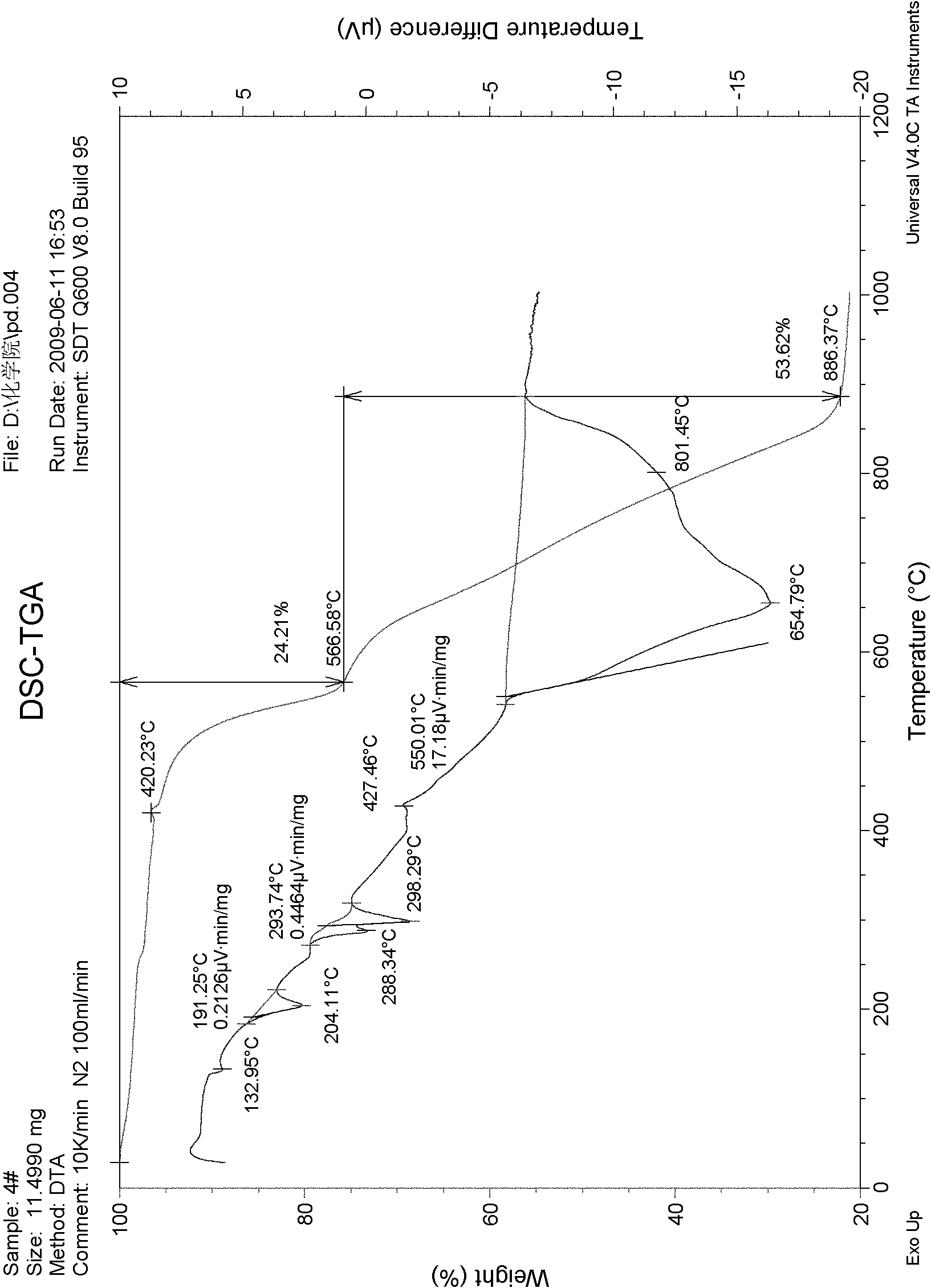
[0067] First, 50% of potassium perchlorate, 10% of potassium nitrate and 40% of barium nitrate are mixed to form a mixture, then tartaric acid accounting for 5% of the total mass of the mixture is added to obtain the oxidant of the present invention, and finally mixed with combustible agent 1:1 to form a firecracker medicament to produce firecrackers. After testing, it was found that:
[0068] The explosion rate is over 95%;
[0069] The firing rate reaches over 98%;
[0070] Sound level: 107.5dB ≤ sound level value should be ≤ 125.2dB;
[0071] The time for the gas product pressure to rise from 0.69MP to 2.07MP is 1.8ms;
[0072] The pH value of the pyrotechnic powder is in the range of 5 to 9;
[0073] Moisture content of pyrotechnic powder ≤1.10%;
[0074] The moisture absorption rate of pyrotechnic powder is ≤2.0%;
[0075] The thermal stability of the pyrotechnic powder is that there is no decomposition phenomenon at 75°C±2°C for 48 hours;
[0076] The friction sens...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com