Method for preparing azobenzene derivatives
A technology of azobenzene derivatives and nitrobenzene derivatives, which is applied in the field of synthesis of azo compounds, can solve problems such as difficult control of reactions, and achieve low cost, simple process, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
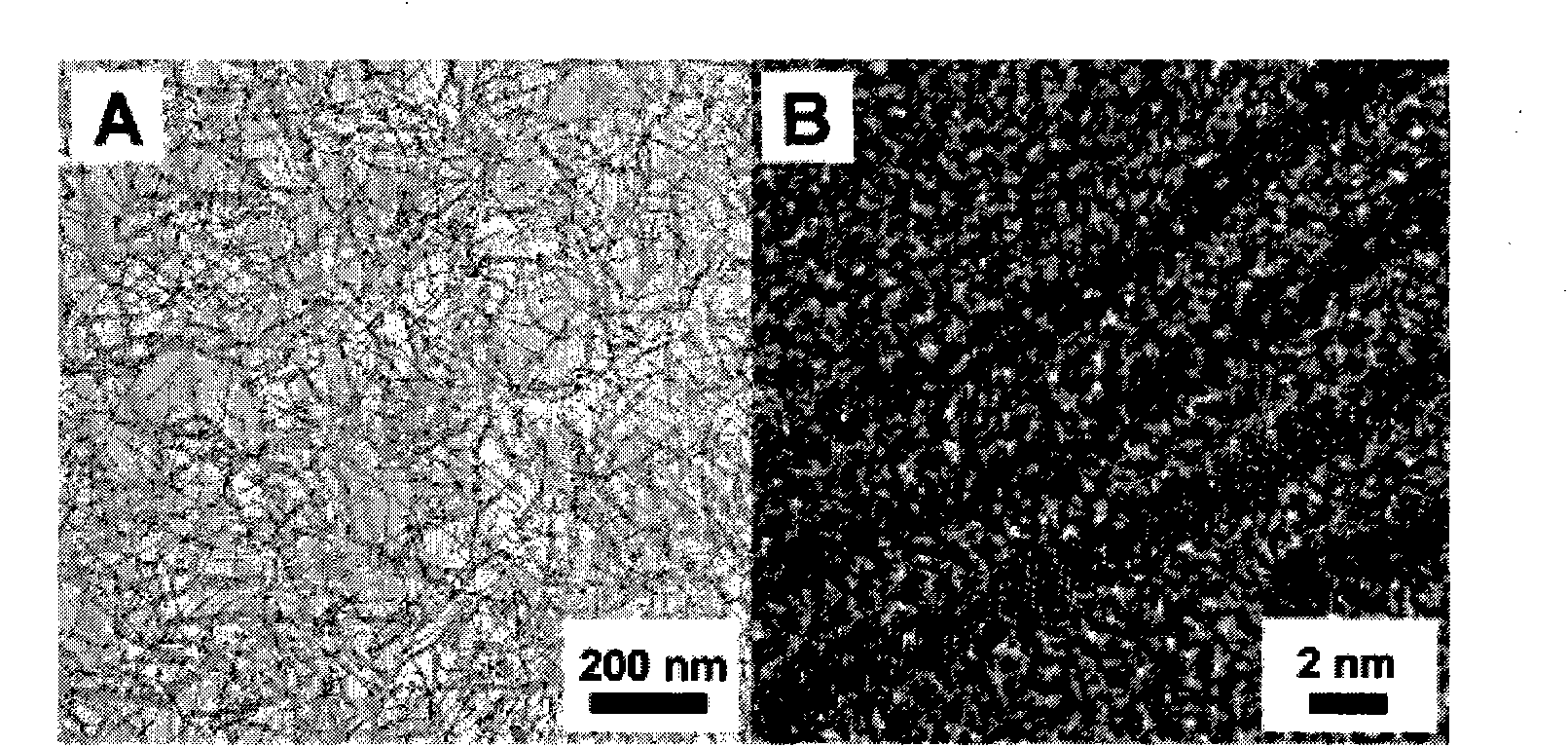
[0019] Example 1: Synthesis of platinum nanowires
[0020] The synthesis method is: reducing platinum acetylacetonate in oleylamine at 160°C and thermally decomposing iron pentacarbonyl to obtain FePt nanowires with a diameter of 2-3nm, and then heating and stirring under acidic conditions to corrode the Fe wrapped around the Pt nanowires. Platinum nanowires were obtained, and the obtained nanowires were scanned by an electron microscope, and the results were as follows figure 1 ,From figure 1 It can be seen that the diameter of the platinum nanowire is 2 to 3 nm.
Embodiment 2
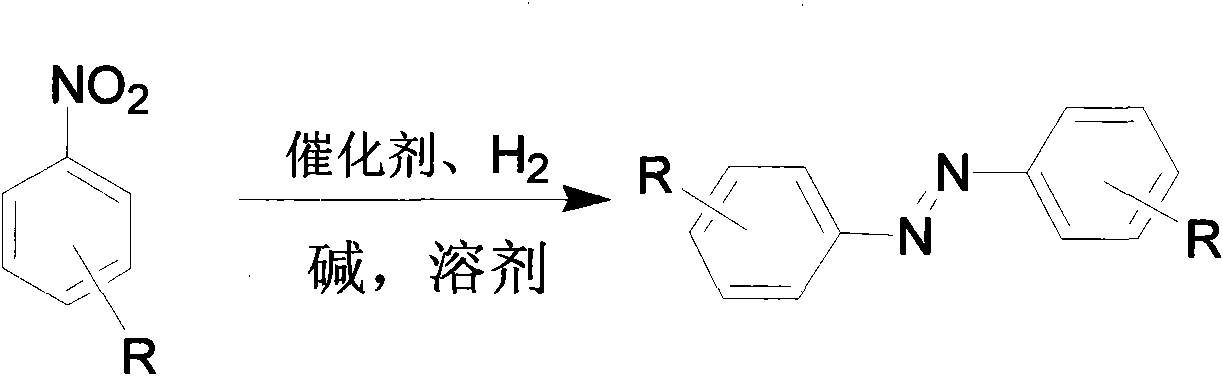
[0021] Embodiment two: if figure 2 As shown, the catalyst catalyzes the preparation of azobenzene derivatives.
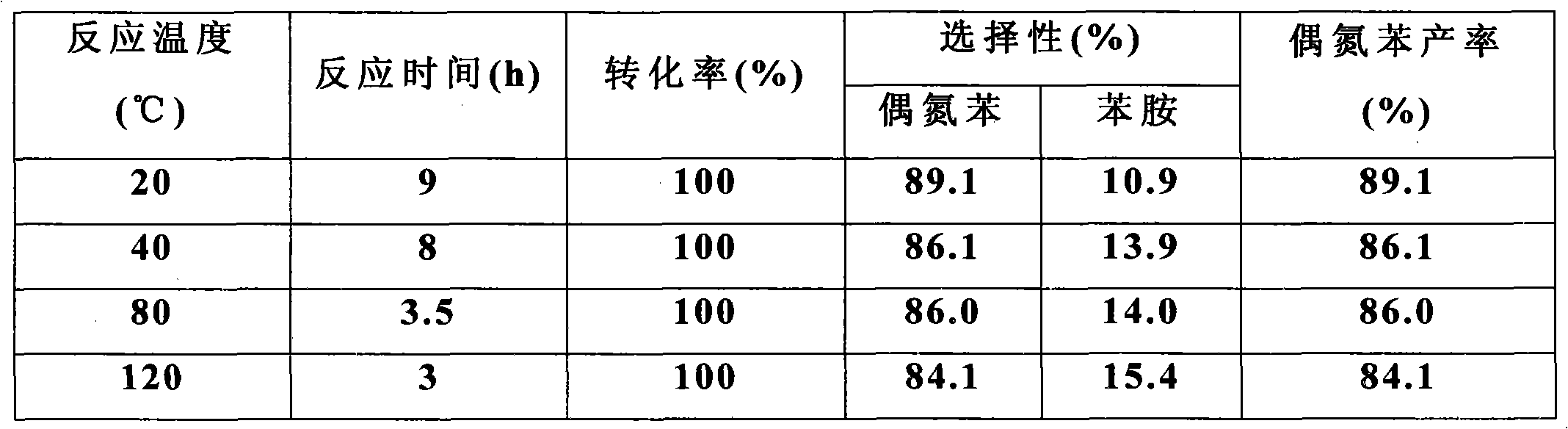
[0022] The platinum nanowire obtained in Example 1 was used as the catalyst, and 0.1 mL of the catalyst (platinum nanowire, about 1 mg), 1 mmol of nitrobenzene, 1 mmol of potassium hydroxide, and 2 mL of p-xylene were added to the reaction tube. The system was connected with a hydrogen bag, then cooled-vacuumized-released hydrogen, cycled 3 to 4 times, put in hydrogen, and returned to room temperature; the system was heated in an oil bath at 20-120°C for 3-9 hours to obtain the product ( Concrete reaction time, reaction temperature are shown in Table 1).
[0023] The product was taken for gas-mass chromatography (GC-MS) and gas chromatography (GC) analysis, and the product was subjected to column separation, and the obtained azo product was characterized by NMR.
[0024] The influence of table 1 different reaction temperature, reaction time on reaction
[0025] ...
Embodiment 3
[0027] Embodiment three: as figure 2 As shown, the catalyst catalyzes the preparation of azobenzene derivatives.
[0028] Using the platinum nanowires obtained in Example 1 as a catalyst, add 0.1 mL of catalyst (platinum nanowires, about 1 mg), 1 mmol of nitrobenzene, 0-2 mmol of potassium hydroxide (see Table 2 for specific dosage), and 2 mL of toluene. The system was connected with a hydrogen bag, then cooled-vacuumized-released hydrogen, cycled 3 to 4 times, put in hydrogen, and returned to room temperature; the system was heated in an oil bath at 80°C for 3.5 hours to obtain the product. The product was analyzed by gas-mass spectrometry (GC-MS) and gas chromatography (GC).
[0029] Table 2 Effect of different potassium hydroxide dosages on the reaction
[0030]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com