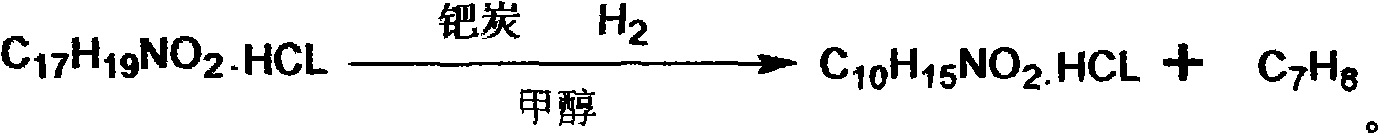Preparation method of etilefrine hydrochloride
A technology of etifolin hydrochloride and benzylethylamino-m-hydroxyacetophenone hydrochloride, applied in the field of preparation of etifolin hydrochloride, can solve the problems of expensive production raw materials, high production cost, problems such as low product purity, to achieve good environmental and economic benefits, less reaction by-products, and shorten the process flow.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
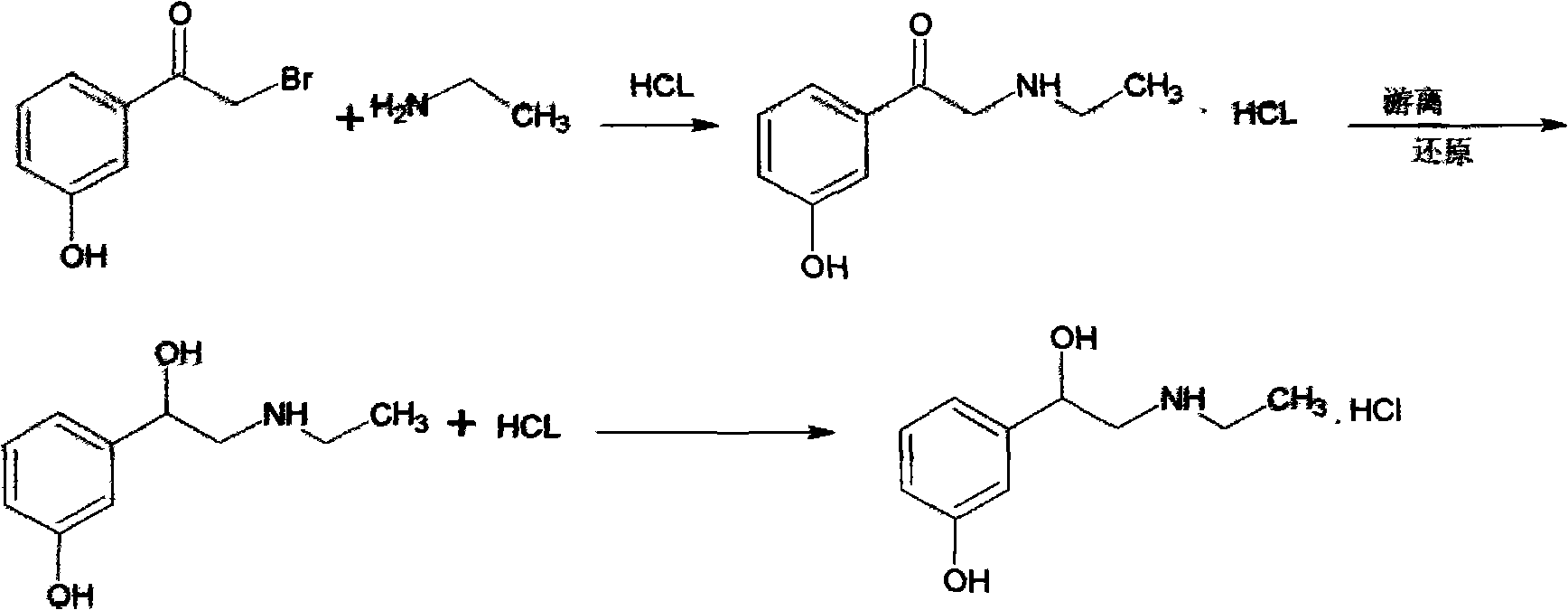
[0032] (1) Amination reaction: α-bromo-m-hydroxyacetophenone is added in the ethanol solution of 50wt% and stirred and dissolved, and the adding weight ratio of the α-bromo-m-hydroxyacetophenone and the ethanol of 50wt% is 1: 8. Continuously and uniformly add N-ethylbenzylamine dropwise at a temperature of 18°C. The feeding ratio of the α-bromo-m-hydroxyacetophenone and N-ethylbenzylamine is 1:1, and keep the reaction for 6 hours Finally, at a temperature of 35°C, add hydrochloric acid solution to adjust the pH value to 1.0, cool down to 10°C, centrifuge and wash with an ethanol solution with a concentration of 96wt%, to obtain α-benzylethylamino-m-hydroxyacetophenone hydrochloride Salt.
[0033] (2) catalytic hydrogenation reaction: α-benzylethylamino m-hydroxyacetophenone hydrochloride is added in 70wt% methanol solution and heated to dissolve, and the α-benzylethylamino m-hydroxyacetophenone hydrochloride is mixed with The adding weight ratio of the methyl alcohol of 70wt%...
Embodiment 2
[0035] (1) Amination reaction: α-bromo-m-hydroxyacetophenone is added in the ethanol solution of 70wt% and stirred and dissolved, and the adding weight ratio of the α-bromo-m-hydroxyacetophenone and 70wt% ethanol is 1: 10. Continuously and uniformly add N-ethylbenzylamine dropwise at a temperature of 16°C. The feeding ratio of the α-bromo-m-hydroxyacetophenone and N-ethylbenzylamine is 1:1.2, and keep the reaction for 4.5 hours Finally, at a temperature of 33°C, add hydrochloric acid solution to adjust the pH value to 1.0, cool down to 10°C, centrifuge and wash with an ethanol solution with a concentration of 95wt%, to obtain α-benzylethylamino-m-hydroxyacetophenone hydrochloride Salt.
[0036] (2) Catalytic hydrogenation reaction: adding α-benzylethylamino m-hydroxyacetophenone hydrochloride into 75wt% methanol solution and heating to dissolve, said α-benzylethylamino m-hydroxyacetophenone hydrochloride The adding weight ratio with the methyl alcohol of 75wt% is 1: 10, after...
Embodiment 3
[0038] (1) Amination reaction: α-bromo-m-hydroxyacetophenone is added in the ethanol solution of 60wt% and stirred and dissolved, and the adding weight ratio of the α-bromo-m-hydroxyacetophenone and 60wt% ethanol is 1: 12. Continuously and uniformly add N-ethylbenzylamine dropwise at a temperature of 15°C, the feeding ratio of the α-bromo-m-hydroxyacetophenone and N-ethylbenzylamine is 1:1.1, and keep the reaction for 5 hours Finally, at a temperature of 34°C, add hydrochloric acid solution to adjust the pH value to 1.0, cool down to 8°C, centrifuge and wash with an ethanol solution with a concentration of 95wt%, to obtain α-benzylethylamino-m-hydroxyacetophenone hydrochloride Salt.
[0039] (2) Catalytic hydrogenation reaction: adding α-benzylethylamino-m-hydroxyacetophenone hydrochloride into 80 wt% methanol solution and heating to dissolve, the α-benzylethylamino-m-hydroxyacetophenone hydrochloride The adding weight ratio with the methanol solution of 80wt% is 1: 12, after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com