Isoflavone glycoside compound and preparation method thereof
A technology of compounds and eluents, applied in the field of biochemistry, to achieve a good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 , Fermentation of Amycolatopsis orientalis ATCC 43491
[0045] Take the slant culture of Amycolatopsis orientalis ATCC 43491, inoculate it into the seed medium for cultivation, and cultivate it on a shaker at 28°C and 220r / min for 40 hours to obtain the seed culture solution.
[0046] The seed culture solution was inoculated into the fermentation medium, and cultured on a shaker at 28° C. at a speed of 220 r / min for 120 hours to obtain a fermentation solution.
[0047] Acidify the fermentation broth with 4mol / L hydrochloric acid, adjust the pH to 3-4, and collect the supernatant by centrifugation.
Embodiment 2
[0048] Example 2 , fermentation broth purification
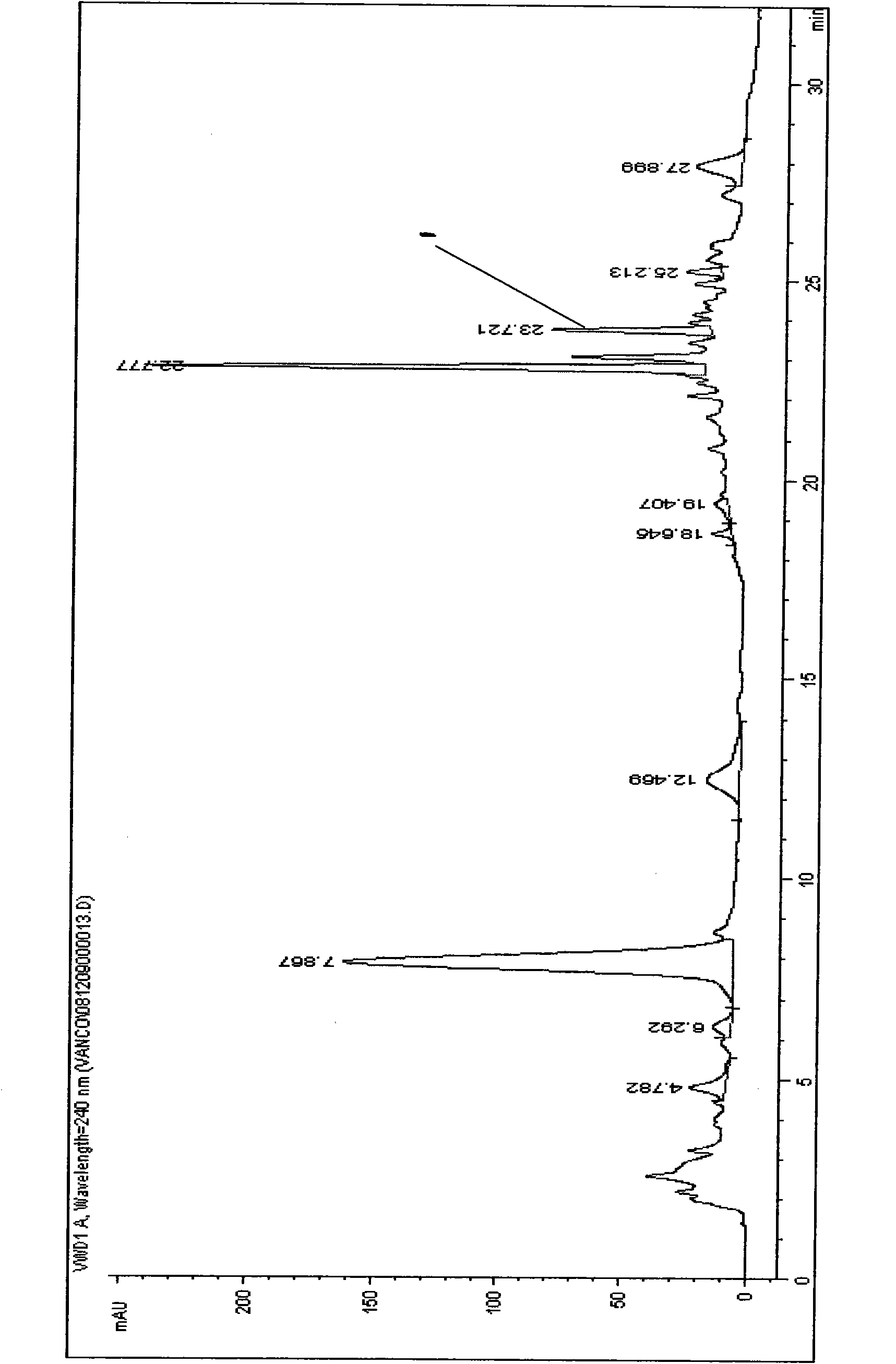
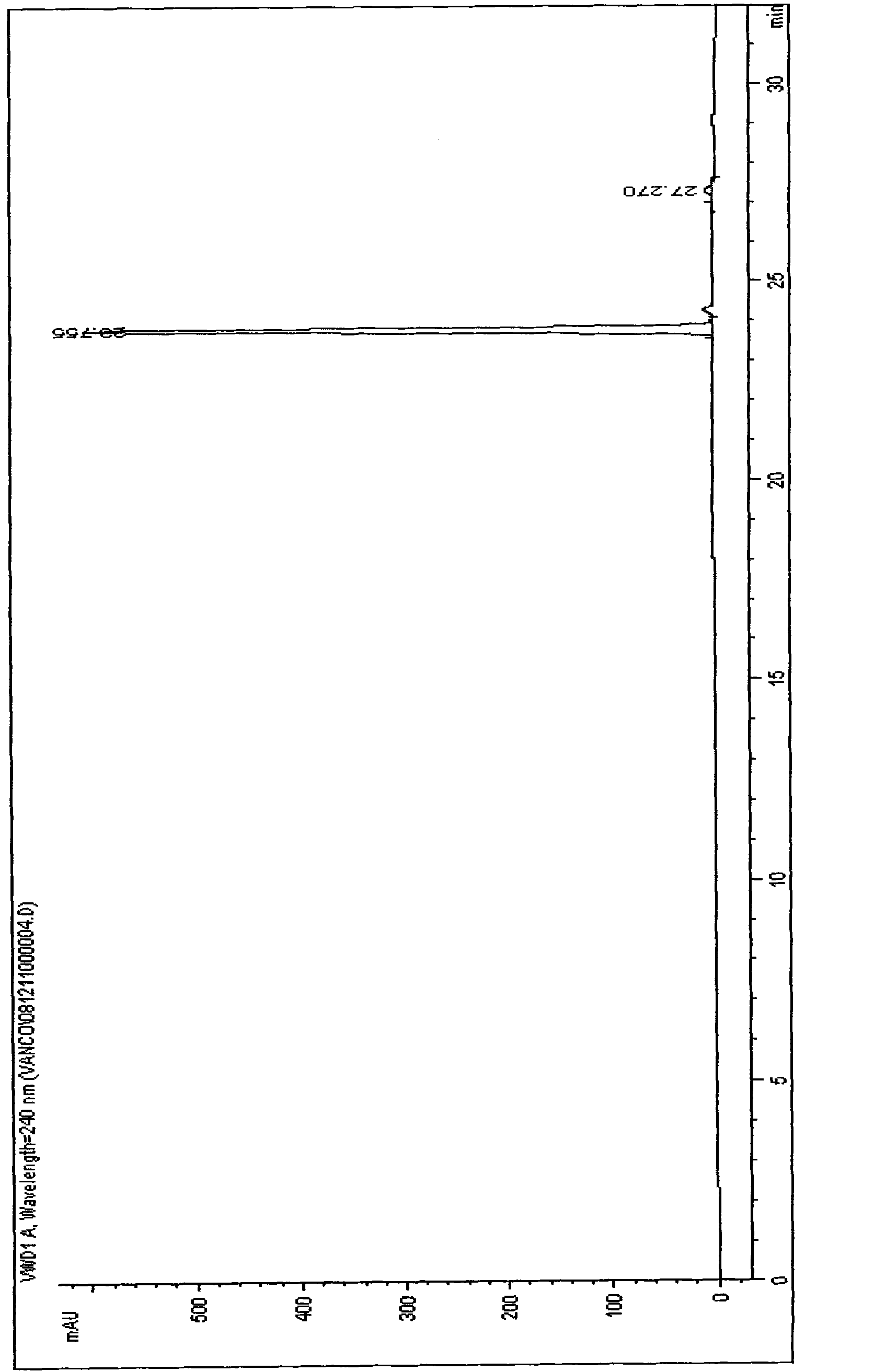
[0049] The centrifugal supernatant that obtains in embodiment 1 is carried out HPLC detection, and the result is as follows figure 1 As shown, then the centrifuged supernatant was purified by HPD100 resin and purified by medium-pressure liquid chromatography to obtain a compound with a purity of 98%. The specific process is as follows:
[0050] A) HPD100 resin primary purification
[0051] The centrifuged supernatant is loaded onto the HPD100 resin column, and ethanol-water solution gradient elution is used to collect the eluate in the range of ethanol volume percentage from 25% to 35%, and then the eluate is concentrated to obtain a crude product concentrate.
[0052] B) the concentrated solution obtained in step A is purified by medium-pressure liquid chromatography technology, and the specific process is as follows:
[0053] The above-mentioned crude product concentrate is loaded on the reversed-phase C-18 column, fir...
Embodiment 3
[0055] Example 3 , LYV09lx01 identification
[0056] The physical and chemical properties of LYV09lx01 are as follows:
[0057] Compound LYV09lx01 is white powder, slightly yellowish, soluble in water, soluble in methanol, insoluble in acetone, butanol, ethyl acetate;
[0058] Mass spectrometry (MS), hydrogen nuclear magnetic resonance ( 1 H NMR), carbon nuclear magnetic resonance ( 13 C NMR) compound LYV09lx01 is measured, and the results are as follows:
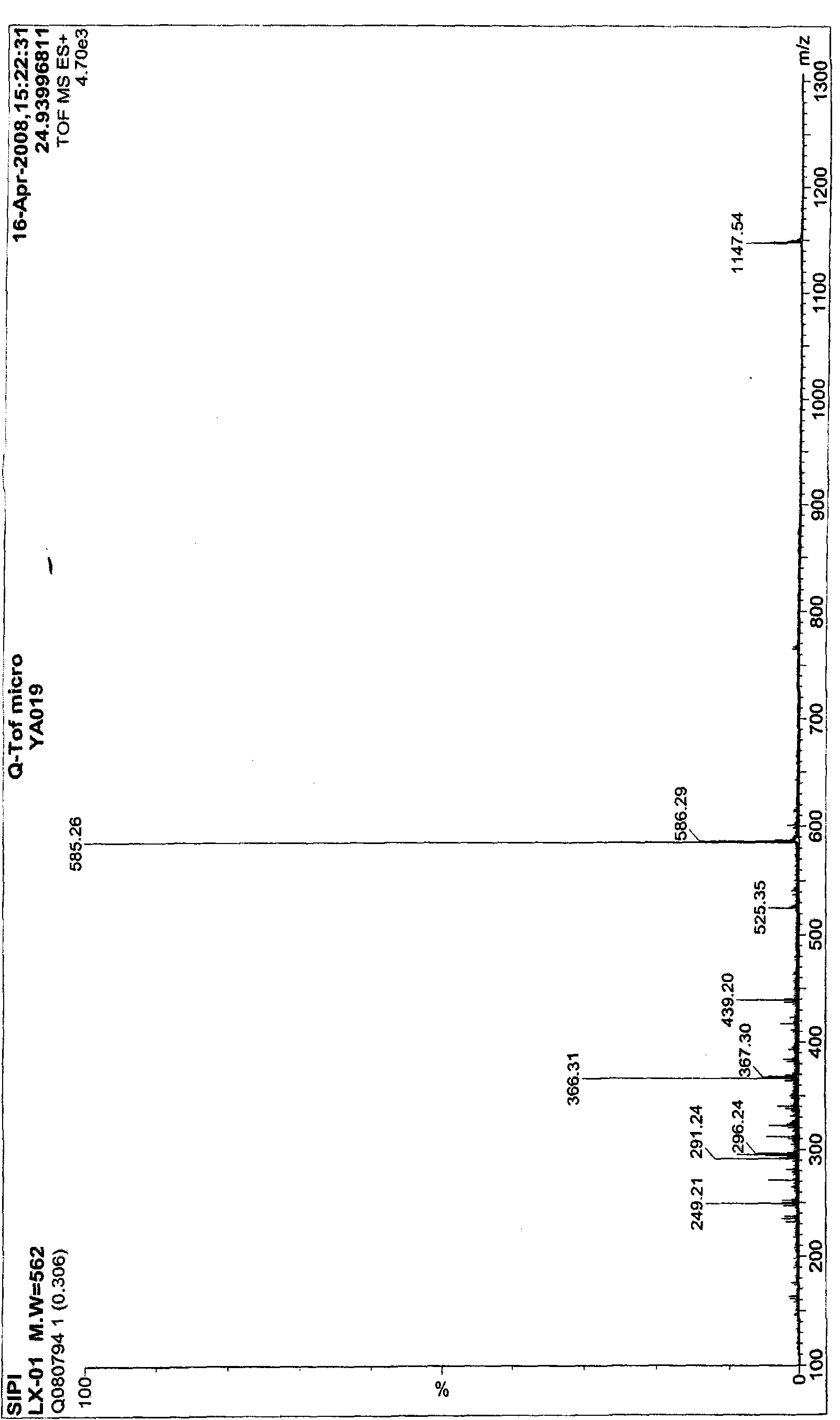
[0059] The mass spectrometry detection results of compound LYV09lx01 are as follows image 3 shown, according to image 3 The result of [M+H] + =563, [2M+H] + = 1125, the molecular weight of compound LYV09lx01 was deduced to be 562.
[0060] The carbon NMR spectrum of compound LYV09lx01 is as Figure 4 As shown, the detection results of its proton nuclear magnetic resonance spectrum are as follows Figure 5 As shown, the spectrum resolution results of the C-NMR spectrum and the H-NMR spectrum are shown in Table 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com