Preparation method and application of Qbeta-2aa phage virus-like particle protein
A granular protein and phage technology, applied in the field of biotechnology drugs and biotherapeutics, can solve the problems of weakened immunity and inability to exert therapeutic effects, and achieve the effects of enhancing immunogenicity, facilitating identification, and treating pathogenic infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
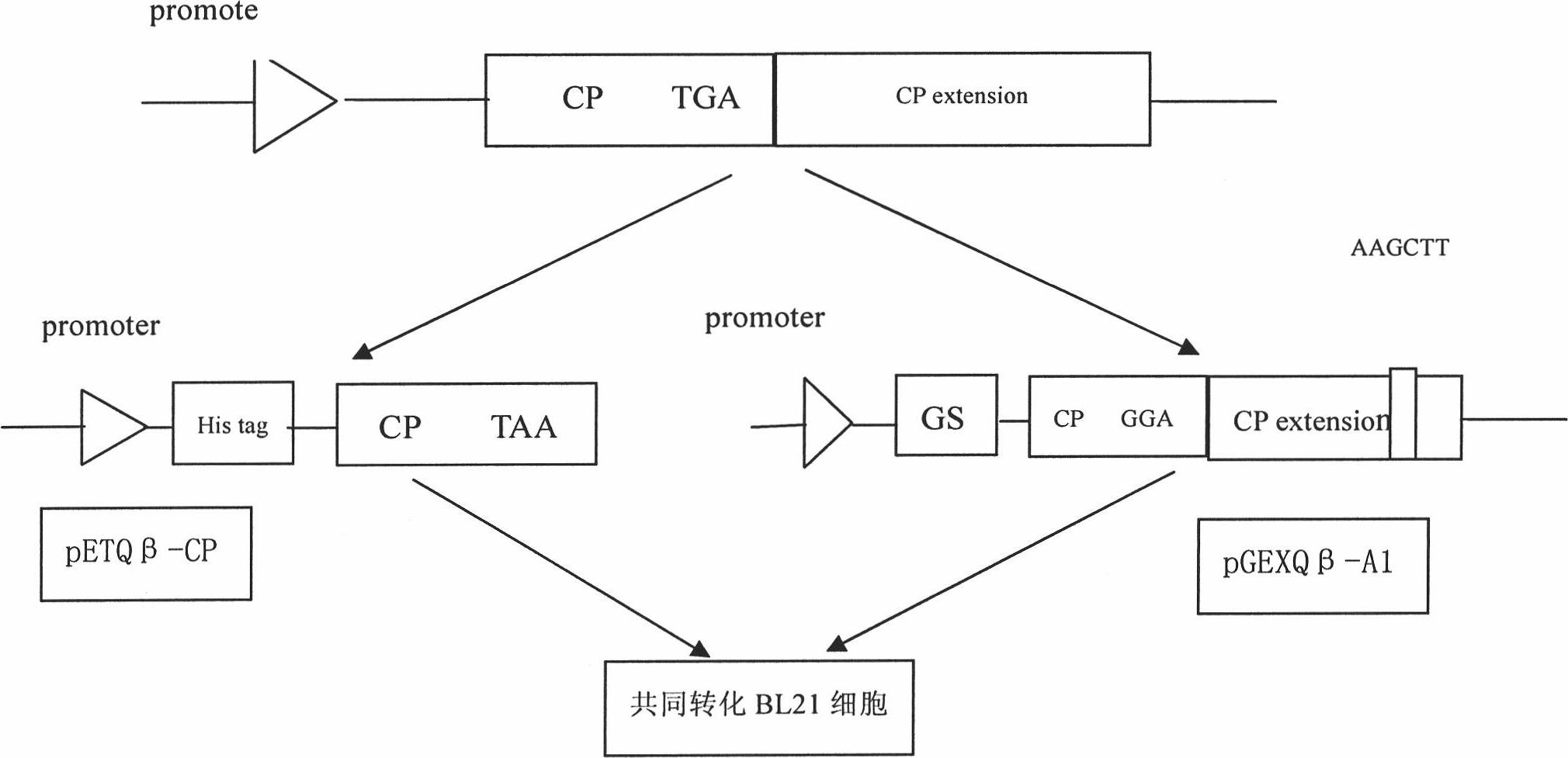
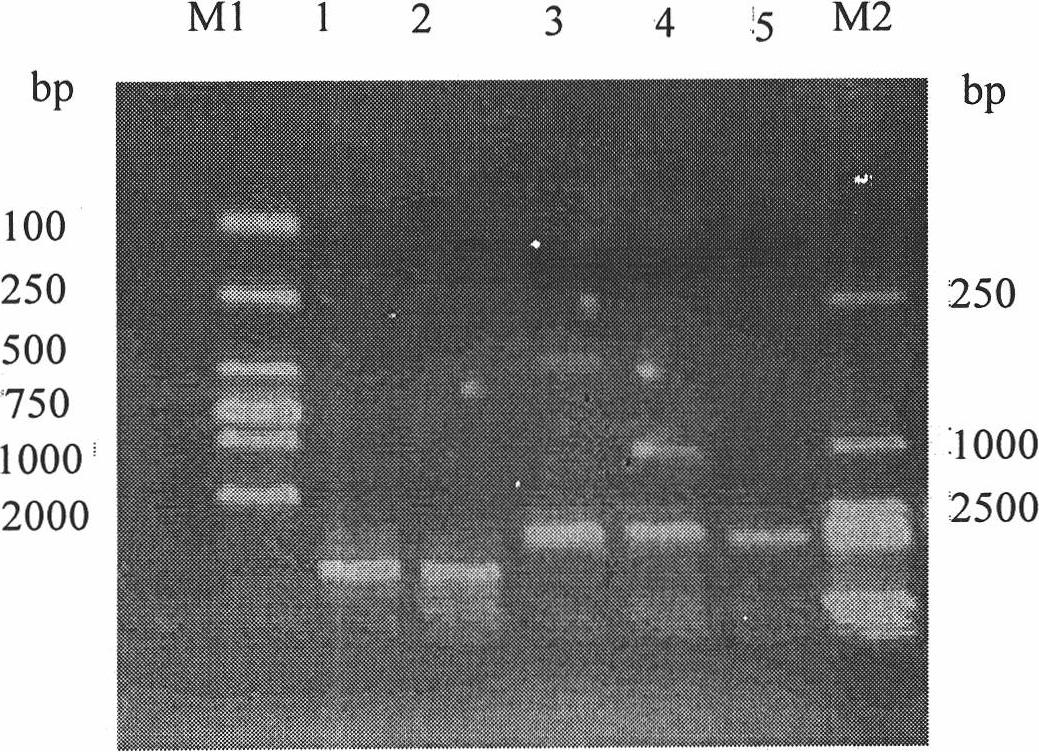
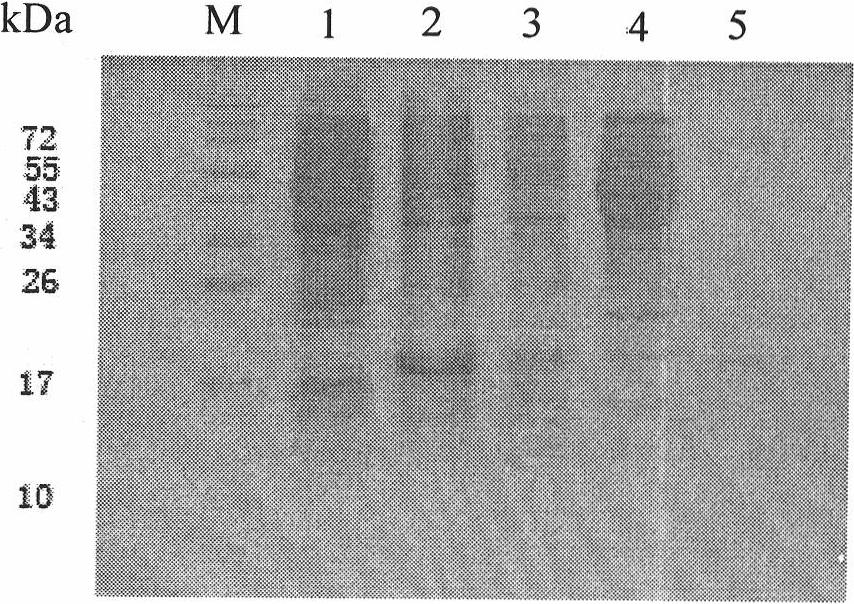
[0034] 1. Cultivation and extraction of Qβ phage
[0035] The Qβ phage strains were purchased from the American Type Culture Collection (ATCC). Refer to the "Guidelines for Molecular Cloning" for recovery and cultivation of phage methods. , 0.1% Glucose, 0.8% NaCl, 0.03% CaCl 2 2H 2 O) dissolve the phage strain, take 0.1ml and dilute several concentrations in proportion. The host strain DH5α was amplified by using LB medium (1% trypticase of casein, 0.5% yeast extract, 0.1% sodium chloride) to make it saturated. Add 1.5% agar powder to the TYG medium, prepare an agar plate after high pressure, and prepare a TYG medium containing 0.5% agar powder in addition, add 500ul saturated host bacteria DH5α after high pressure and wait until the temperature drops to about 30°C, and pour it into The 1.5% agar plate has been solidified. After solidification, drop different dilutions of Qβ bacteriophage onto the plate, overnight at 37°C, and the formation of phage plaques can be seen the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com