Recombinant BCG vaccine rBCG::X
A technology of recombinant BCG vaccine and BCG vaccine is applied in the fields of genetic engineering vaccines and tuberculosis vaccines, which can solve problems such as difficulty in obtaining HspX protein, and achieve the effects of strong immune protection type and strong protection effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
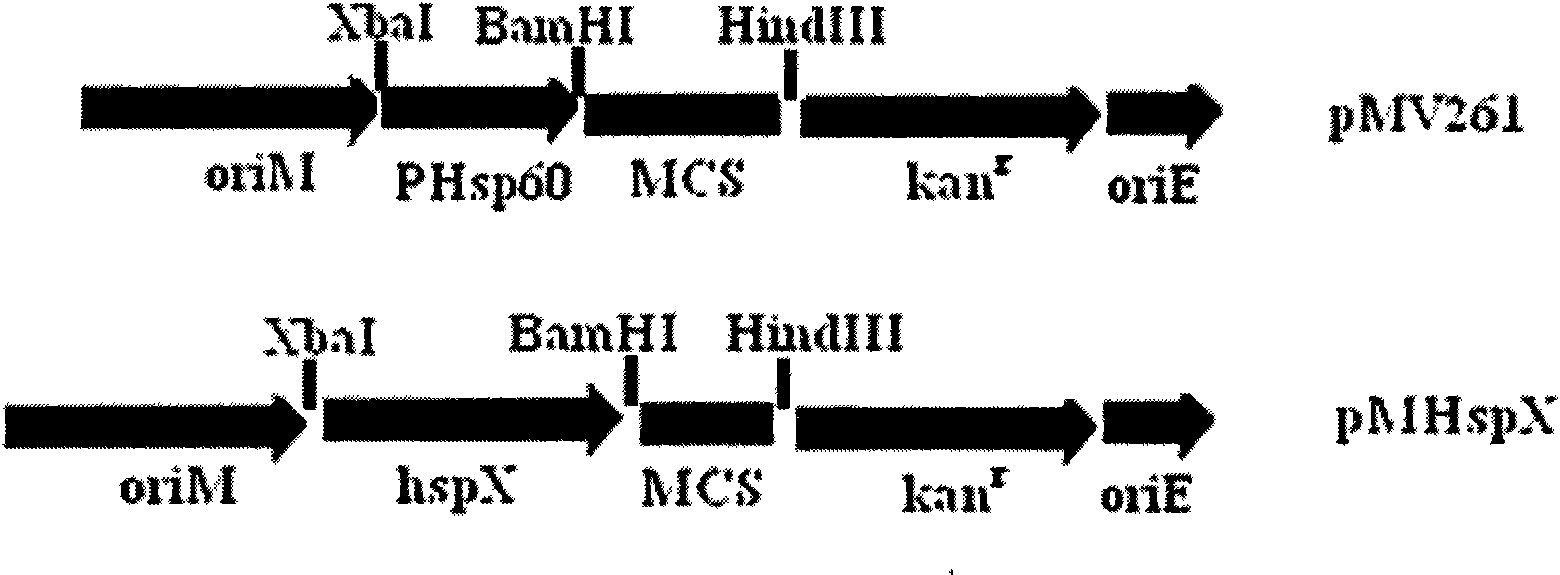
[0038] Construction and Identification of Recombinant Plasmid pMHspX
[0039] The molecular biology technique was carried out according to the routine: the 0.8kb HspX (acr, Rv2031c) gene was amplified from the genome of Mycobacterium tuberculosis H37Rv by PCR technique. The amplification conditions were as follows: 95°C for 5 minutes, then 94°C for 45s, 60°C for 45s, 72°C for 50s, 30 cycles, and finally 72°C for 5 minutes. PCR products were recovered using the AxyPrep PCR Product Recovery Kit (Axygen). After digesting the 0.8kb acr gene with BamHI and XbaI, it was recovered with AxyPrep DNA Gel Extraction Kit (Axygen). Then the acr gene was ligated with the pcDNA3.1(-) recovered by the same enzyme digestion, and then subcloned into the Escherichia coli-mycobacterium shuttle plasmid pMV261 to form the recombinant plasmid pMHspX. Further enzyme digestion identification and sequence analysis proved that the constructed recombinant expression plasmid was completely correct. Seq...
Embodiment 2
[0041] Establishment of Recombinant BCG rBCG::X
[0042] The preparation of recombinant BCG rBCG::X is as follows. First prepare the competent state of BCG. Take 1ml of BCG strain in the logarithmic growth phase, inoculate aseptically in 50ml of 7H9 liquid medium, and culture at 37°C for 2 weeks. After cooling the medium on ice for 2 hours, the bacteria were collected by centrifugation at 4°C. Add 1ml of 10% ice-cold glycerin to resuspend, and disperse the bacteria with sticky grinder. After that, wash 3 times with 1 / 2, 1 / 10 and 1 / 50 of the volume of the original culture volume of ice-cold glycerol, and finally resuspend with 1ml of ice-cold glycerol, aliquot into 100μl tubes, and store at -80°C for later use. The second is the electroporation of BCG. First, add 100 μl of competent BCG strain to a pre-cooled 2mm Bio-Rad electroporation cup, add the purified recombinant plasmid pMHspX (less than 5 μl), mix well, and ice-bath for 10 minutes, remove the air bubbles in the cup...
Embodiment 3
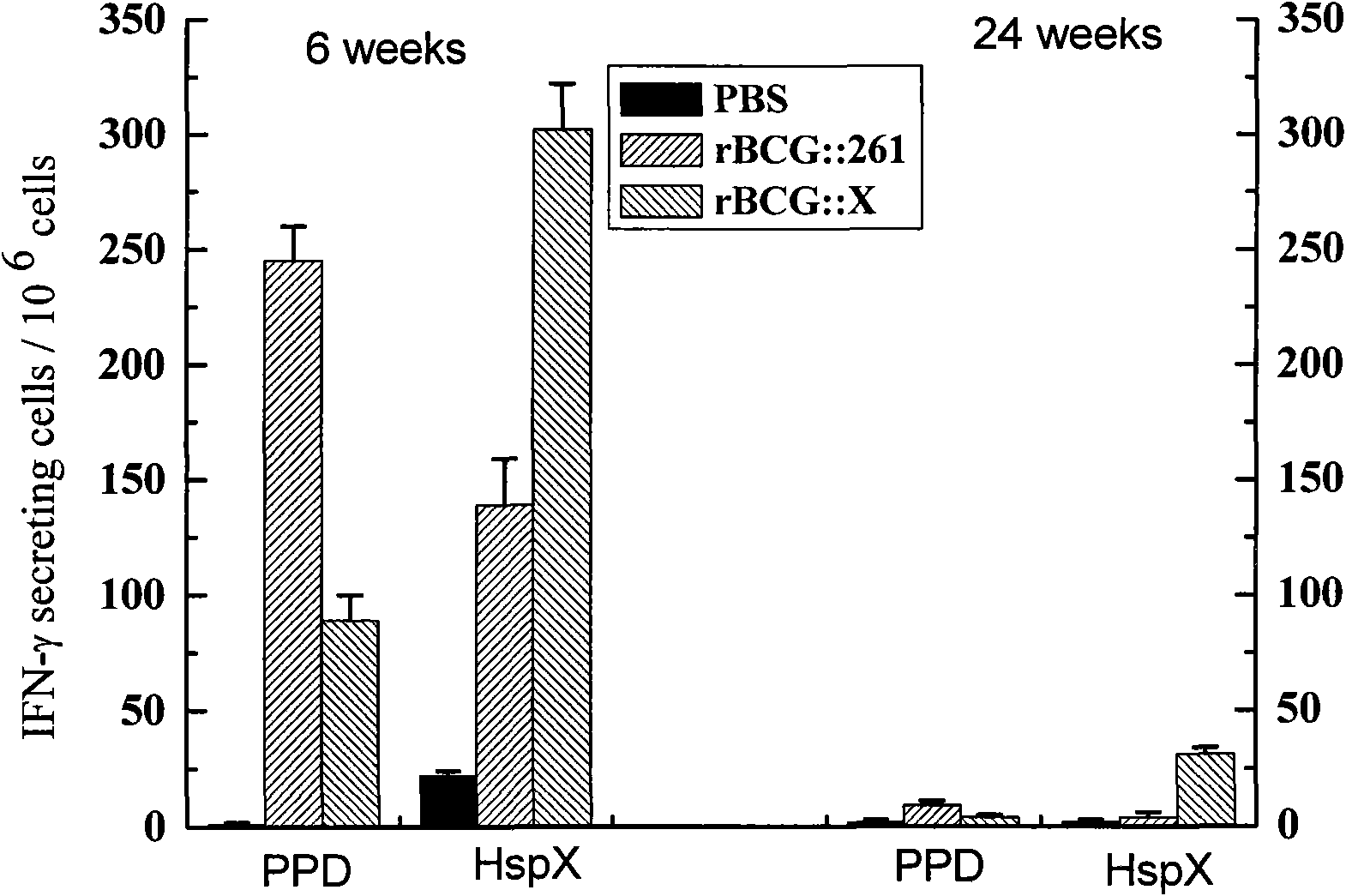
[0044] Cellular Immunity Characteristics of Recombinant BCG rBCG::X
[0045] 10 respectively 6 CFU recombinant vaccines rBCG::X and rBCG::261 were used to immunize C57BL / 6 mice, and PBS was used as control. Six weeks and 24 weeks after the mice were immunized, the spleen lymphocytes of the immunized mice were aseptically isolated, and the number of secreted IFN-γ cells specific to the spleen lymphocyte antigen was detected by ELISPOT technique. Antigens include PPD, Ag85B and HspX proteins, the antigen concentration is 2 μg / ml, and the number of cells is 10 6 . See image 3 , the recombinant vaccine rBCG::X induced an increase in the number of secreted IFN-γ cells specific to the HspX protein in the spleen lymphocytes of short-term and long-term immunized mice.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com