Nucleotide sequence, method and kit for detecting human influenza A virus
An influenza A virus, nucleotide sequence technology, applied in the directions of microorganism-based methods, biochemical equipment and methods, and microorganism determination/inspection, etc., can solve problems such as difficult specific diagnosis, false positives, cross-reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
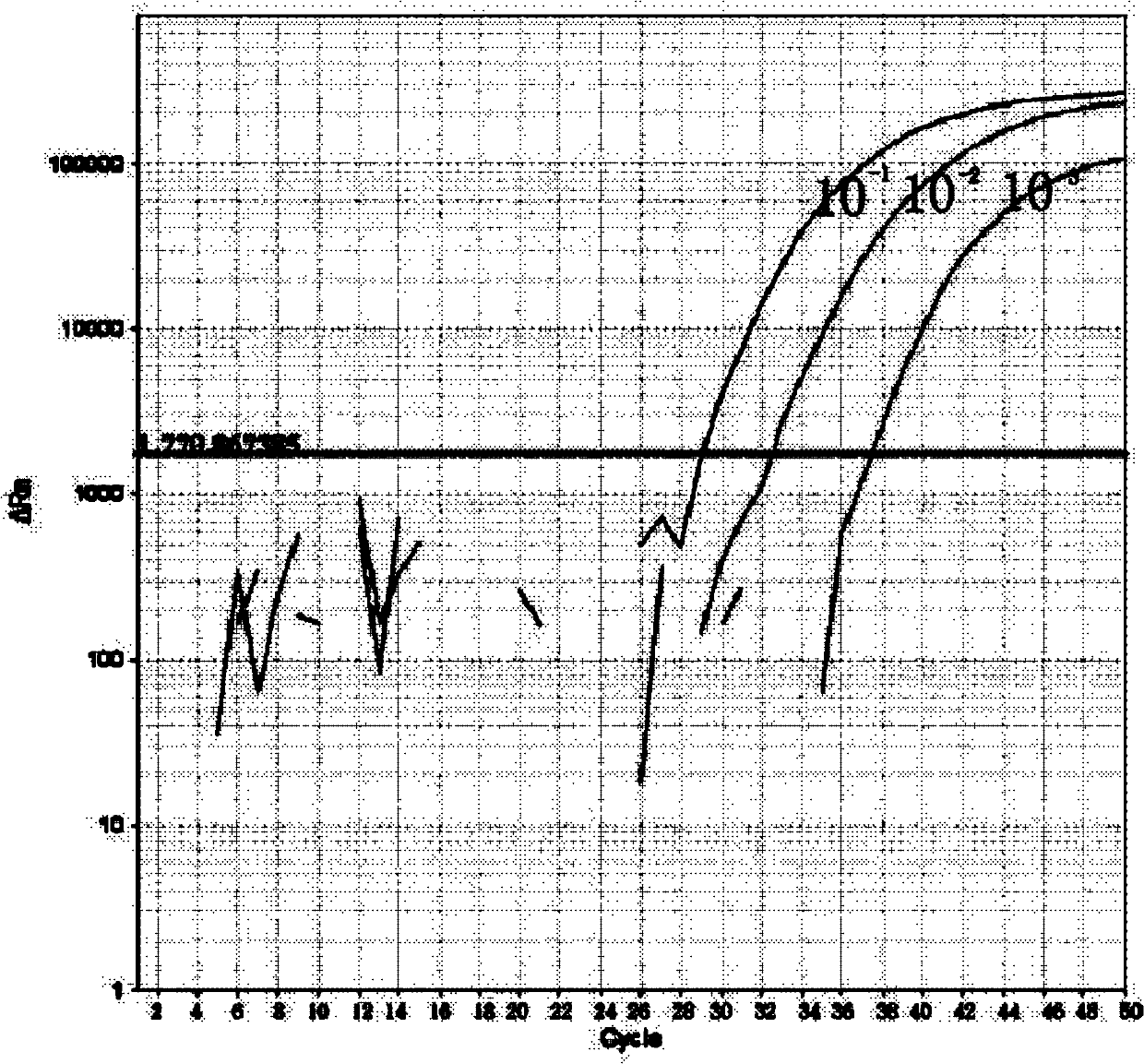
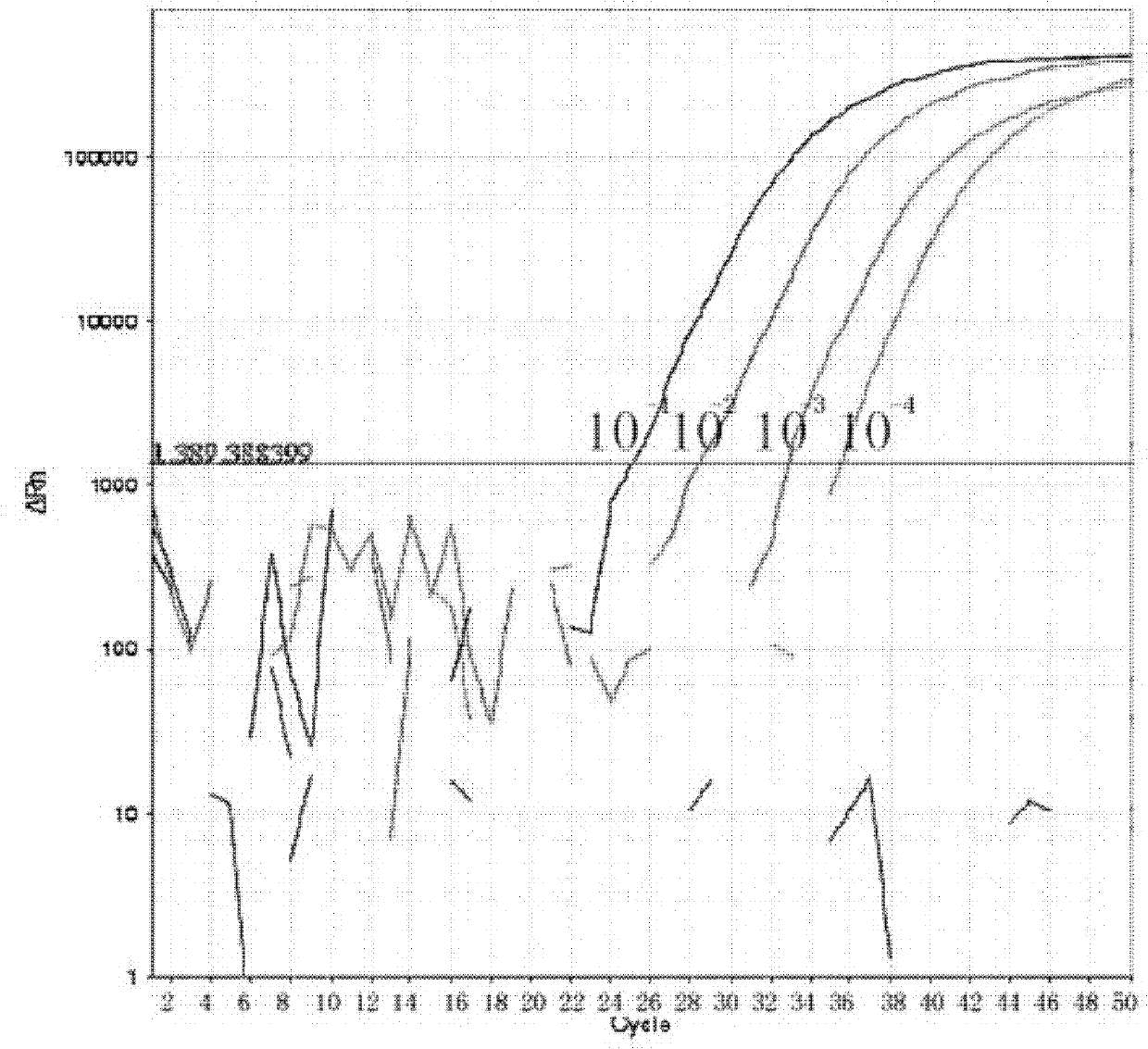
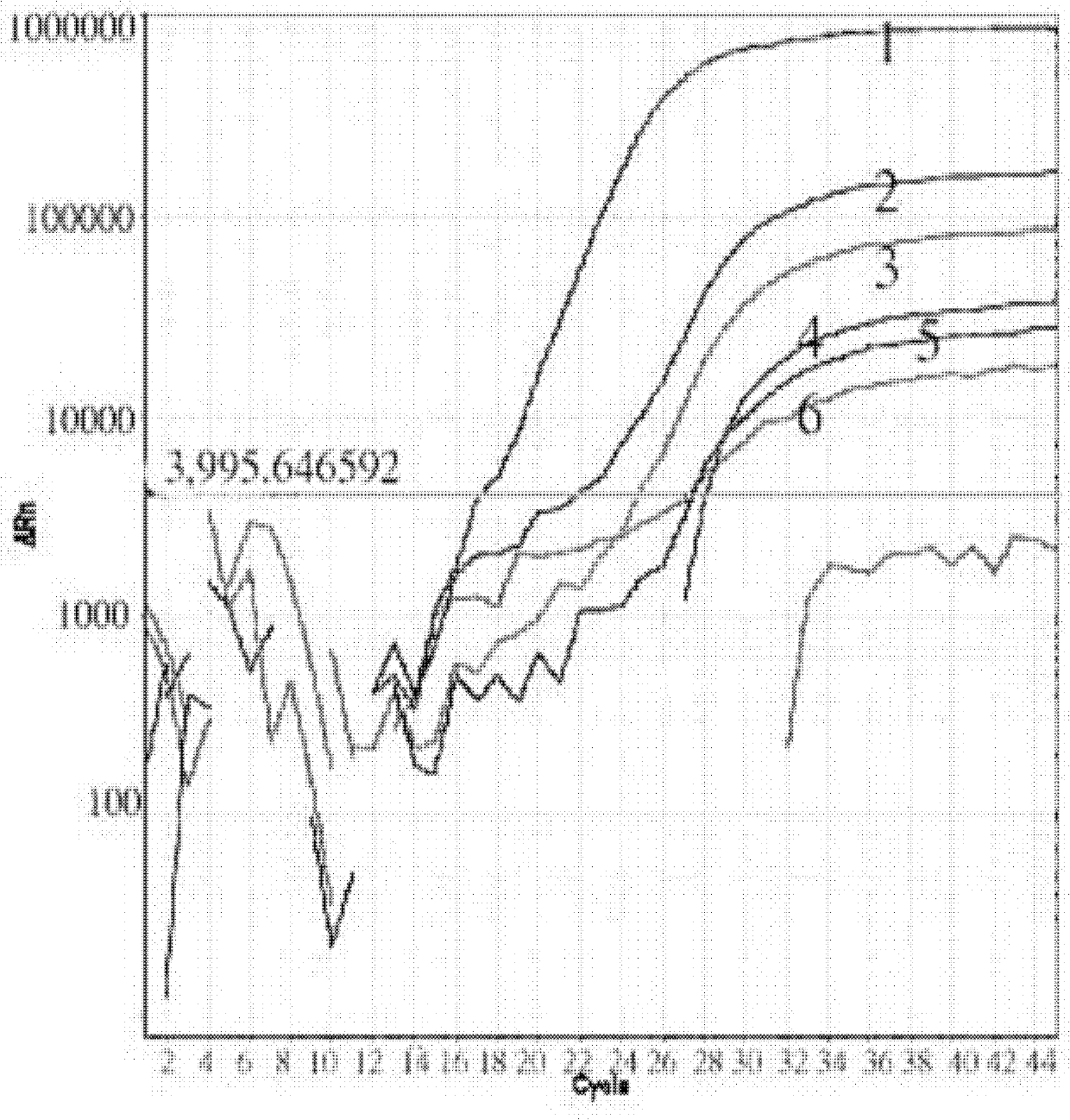
Image
Examples
Embodiment 1
[0062] Example 1 Influenza A virus, seasonal influenza A virus and new type A H1N1 influenza virus typing detection method and kit
[0063] 1: material
[0064] 1.1 Virus samples:
[0065] 1 swine influenza virus sample (virus sample from: Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences);
[0066] 1 sample of the new epidemic influenza A (H1N1) virus (virus sample from: Hong Kong Queen Mary Hospital);
[0067] 3 samples of seasonal influenza A virus: 1 seasonal influenza A H1, 1 seasonal influenza A H3, 1 seasonal influenza A H9 (virus samples from: Guangzhou Center for Disease Control and Prevention) ;
[0068] 1 highly pathogenic H5N1 avian influenza virus sample (virus sample from: Hong Kong Queen Mary Hospital);
[0069] 1 copy of influenza B virus (virus sample from: Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences).
[0070] 2. Method
[0071] 2.1 Acquisition of total RNA of the virus strain
[0072]...
Embodiment 2
[0089] Example 2 Kit
[0090] The specification of this kit is for 20 people, and the composition of the kit is:
[0091]
[0092] Among them, RT-PCR reaction solution: 100 μl of 10× reverse transcription-PCR reaction solution, 40 μl of 10 mM dNTP, 200 μl of 5× reverse transcription enhancement solution, 10 μl of RNase inhibitor, 20 μl of 20 μM primers and probes (SEQ ID NO: 1 -12), to 800μl, mix well.
[0093] Mixed enzyme solution: 10 μl reverse transcriptase and 30 μl hot-start DNA polymerase.
[0094] The positive control substance is a solution containing plasmids pMD19A, pMD19H1 and pMD19RnaseP, the new type A H1N1 quantitative reference substance is a solution containing plasmid pMD19H1N1, and the negative control substance is the oral secretion of normal people.
Embodiment 3
[0095] The usage method of embodiment 3 kit:
[0096] 1. Reagent Preparation
[0097] 1.1 Thaw the reagent completely before opening the tube, mix well and centrifuge briefly
[0098] 1.2 Preparation: Calculate by taking 43 μl of RT-PCR reaction solution and 2 μl of mixed enzyme for each reaction, multiply by the number of reaction tubes required (it is recommended to add one more reaction volume as appropriate), add them to a total sterile centrifuge tube, Vortex to mix.
[0099] 1.3 Dispensing: Add 45 μl of the above-mentioned mixed reaction solution into each PCR reaction tube with a micro-sampler, and store at 4°C.
[0100] Note: It is best to prepare the reaction solution after the RNA extraction is completed.
[0101] 2. Sample RNA extraction: Use Qiagen's RNA kit to process the sample, extract and purify the total RNA, dissolve it in 50ul DEPC water, and follow the manufacturer's instructions for the entire operation.
[0102] 3. Adding samples: Add 5ul of the sampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com