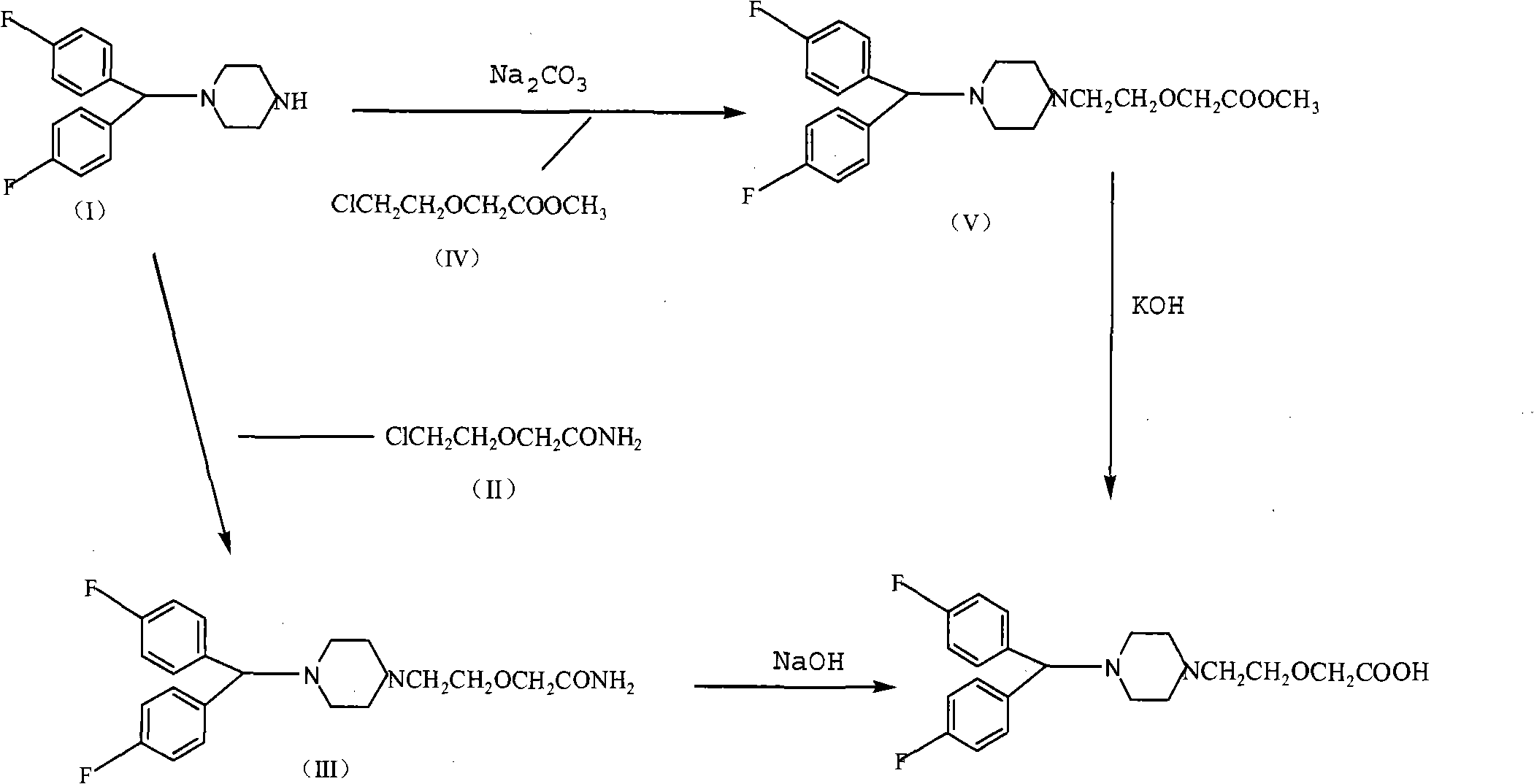
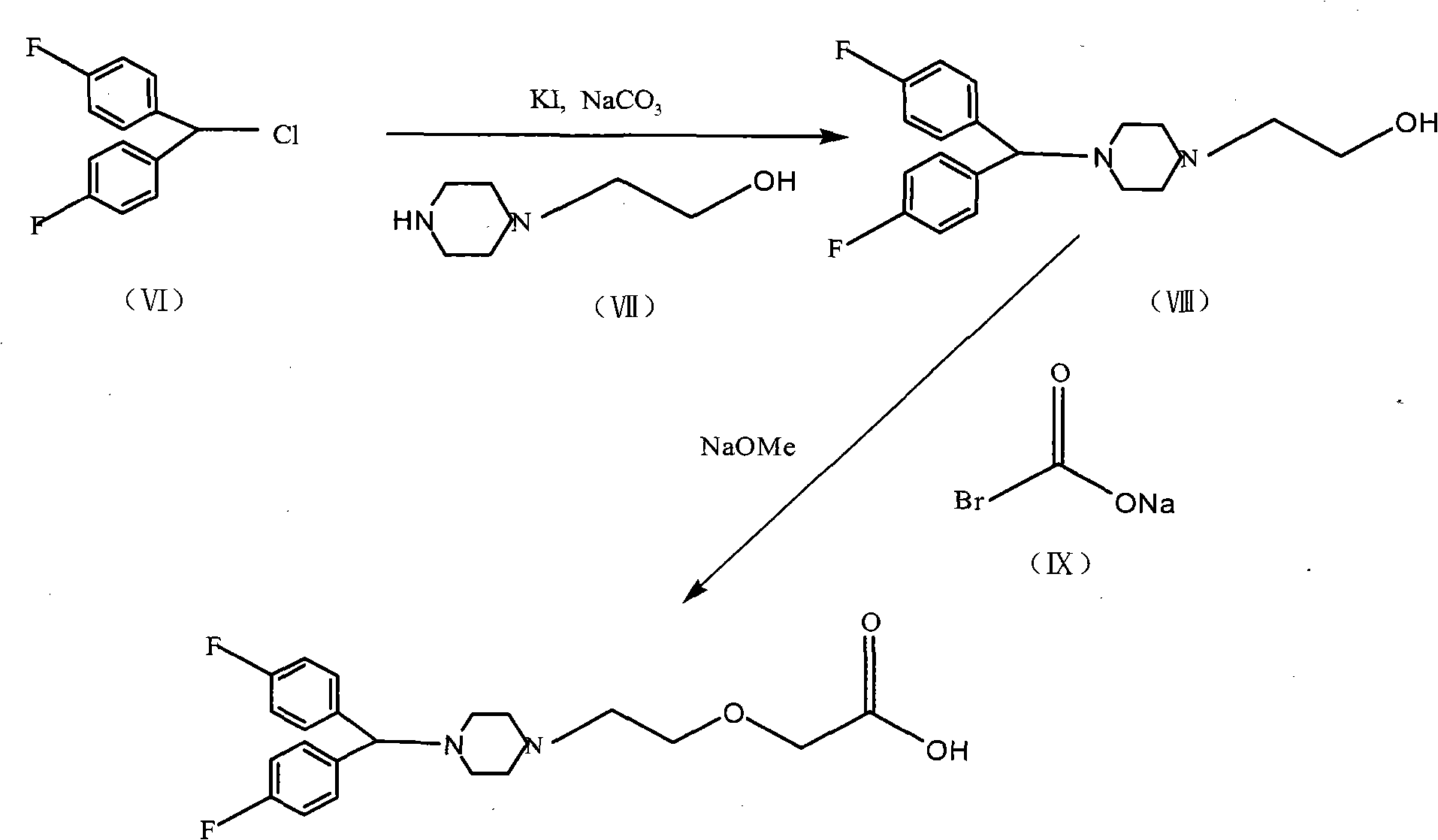
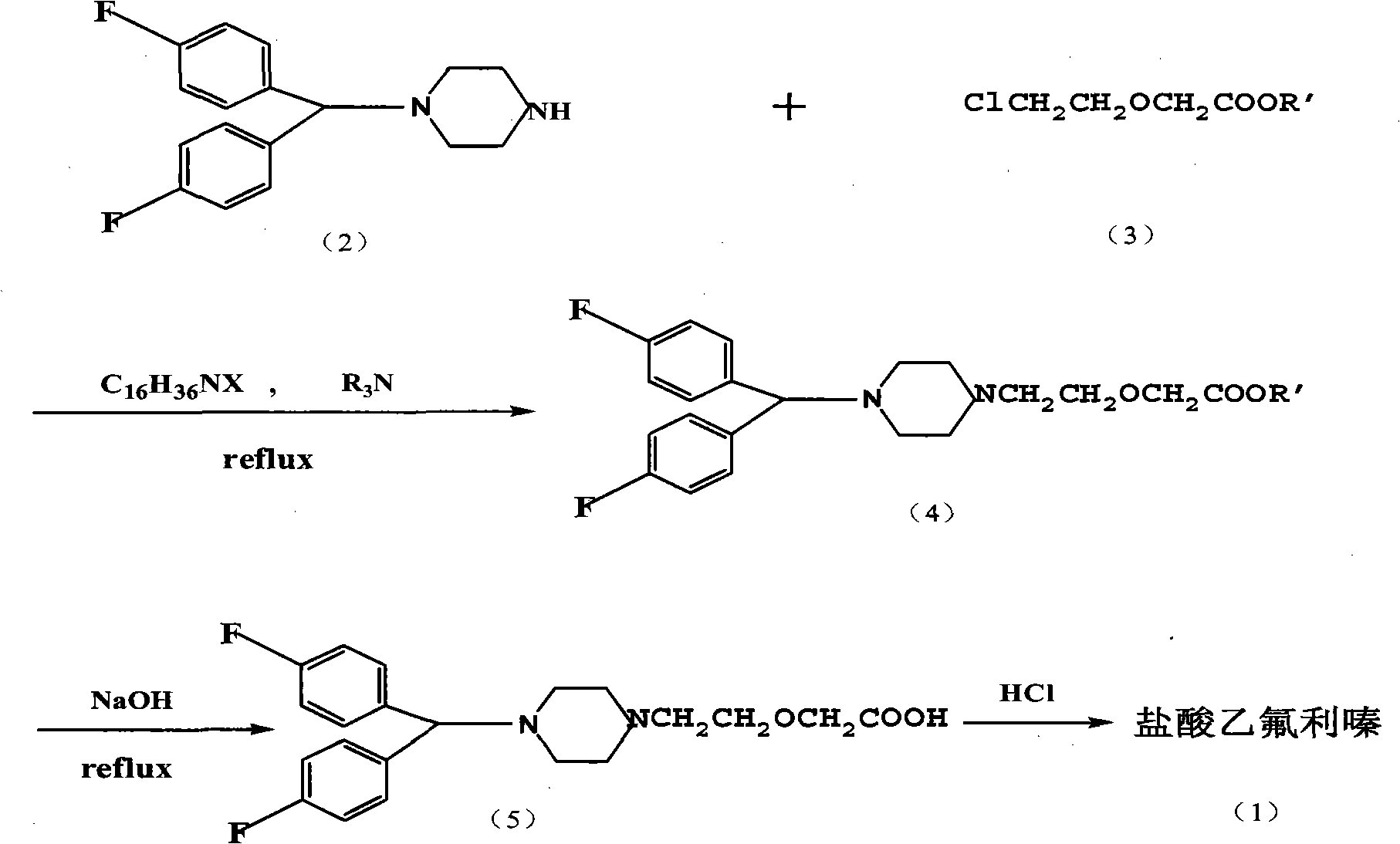
Method for preparing efletirizine dihydrochloride
A technology of eflurazine hydrochloride and fluorophenyl, which is applied in the field of preparation of eflurazine hydrochloride, can solve the problems of cumbersome steps and low price of raw material compounds, and achieve the effects of simple raw materials, low cost and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of ethyl 2-[2-[4-[bis(4-fluorophenyl)methyl]-1-piperazinyl]ethoxy]acetate
[0036] 1-[bis(4-fluorophenyl)methyl]piperazine 5.74g (0.02mol), ethyl chloroethoxyacetate 3.34g (0.02mol), triethylamine 4.04g (0.04mol), xylene 30ml , Heated to reflux, added 0.67 g (0.004 mol) of ethyl chloroethoxyacetate after 10 h of reaction and continued to reflux for 8 h. Stop the reaction, filter with suction, wash the filter cake with 20 ml of hot toluene, rotate the filtrate, and directly hydrolyze the residue without purification to obtain eflurizine hydrochloride. Yield 68.5%.
Embodiment 2
[0038] Preparation of Methyl 2-[2-[4-[bis(4-fluorophenyl)methyl]-1-piperazinyl]ethoxy]acetate
[0039] 1-[bis(4-fluorophenyl)methyl]piperazine 5.74g (0.02mol), methyl chloroethoxyacetate 3.05g (0.02mol), triisopropylamine 5.72g (0.04mol), tetrabutyl Ammonium iodide 0.07g (0.2mmol), xylene 30ml, heated to reflux, reacted for 10h, added 0.61g (0.004mol) of ethyl chloroethoxyacetate and continued to reflux for 8h. Stop the reaction, filter with suction, wash the filter cake with 20 ml of hot toluene, rotate the filtrate, and directly hydrolyze the residue without purification to obtain eflurizine hydrochloride. Yield 80.2%.
Embodiment 3
[0041] Preparation of ethyl 2-[2-[4-[bis(4-fluorophenyl)methyl]-1-piperazinyl]ethoxy]acetate
[0042] 1-[bis(4-fluorophenyl)methyl]piperazine 5.74g (0.02mol), ethyl chloroethoxyacetate 3.34g (0.02mol), potassium iodide 0.033g (0.2mmol), triethylamine 4.04g (0.04mol), xylene 30ml, heated to reflux, after 10h of reaction, added 0.67g (0.004mol) of methyl chloroethoxyacetate and continued to reflux for 8h. Stop the reaction, filter with suction, wash the filter cake with 20 ml of hot toluene, rotate the filtrate, and directly hydrolyze the residue without purification to obtain eflurizine hydrochloride. Yield 75.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com