Human source antibody and humanization remolding method thereof
A humanized antibody and antibody technology, applied in chemical instruments and methods, biochemical equipment and methods, antibodies, etc., can solve the problems of complex high-affinity antibody methods, time-consuming and labor-intensive methods, and different antibodies are not optimal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1. Humanized transformation of chimeric antibody chA21
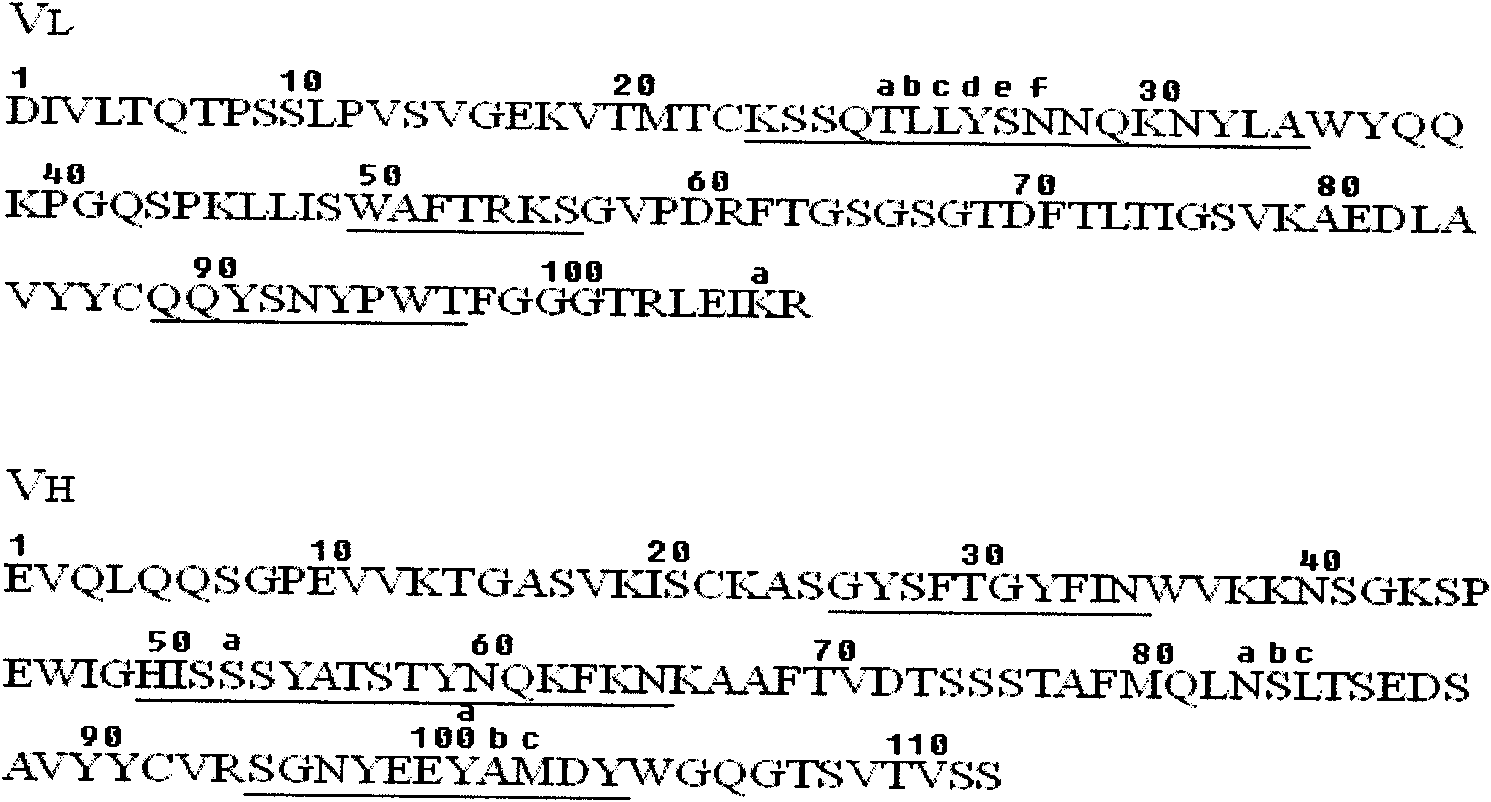
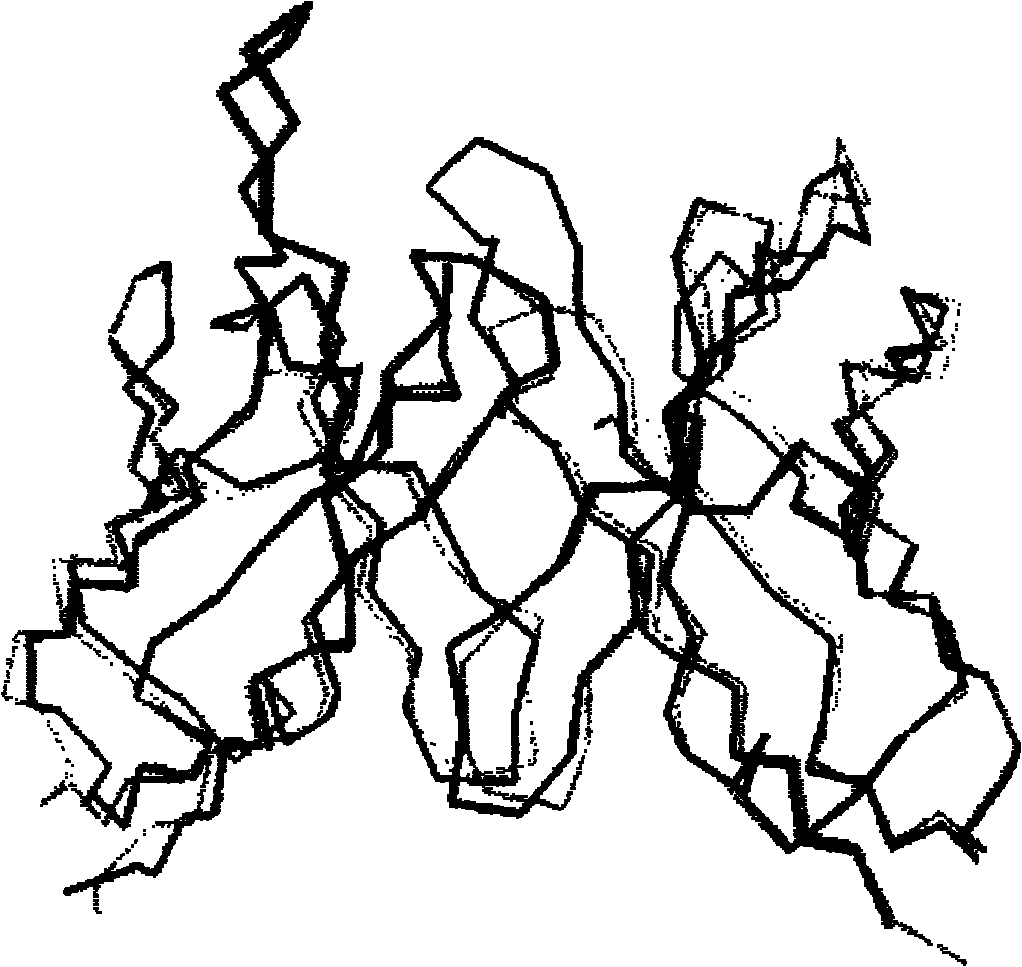
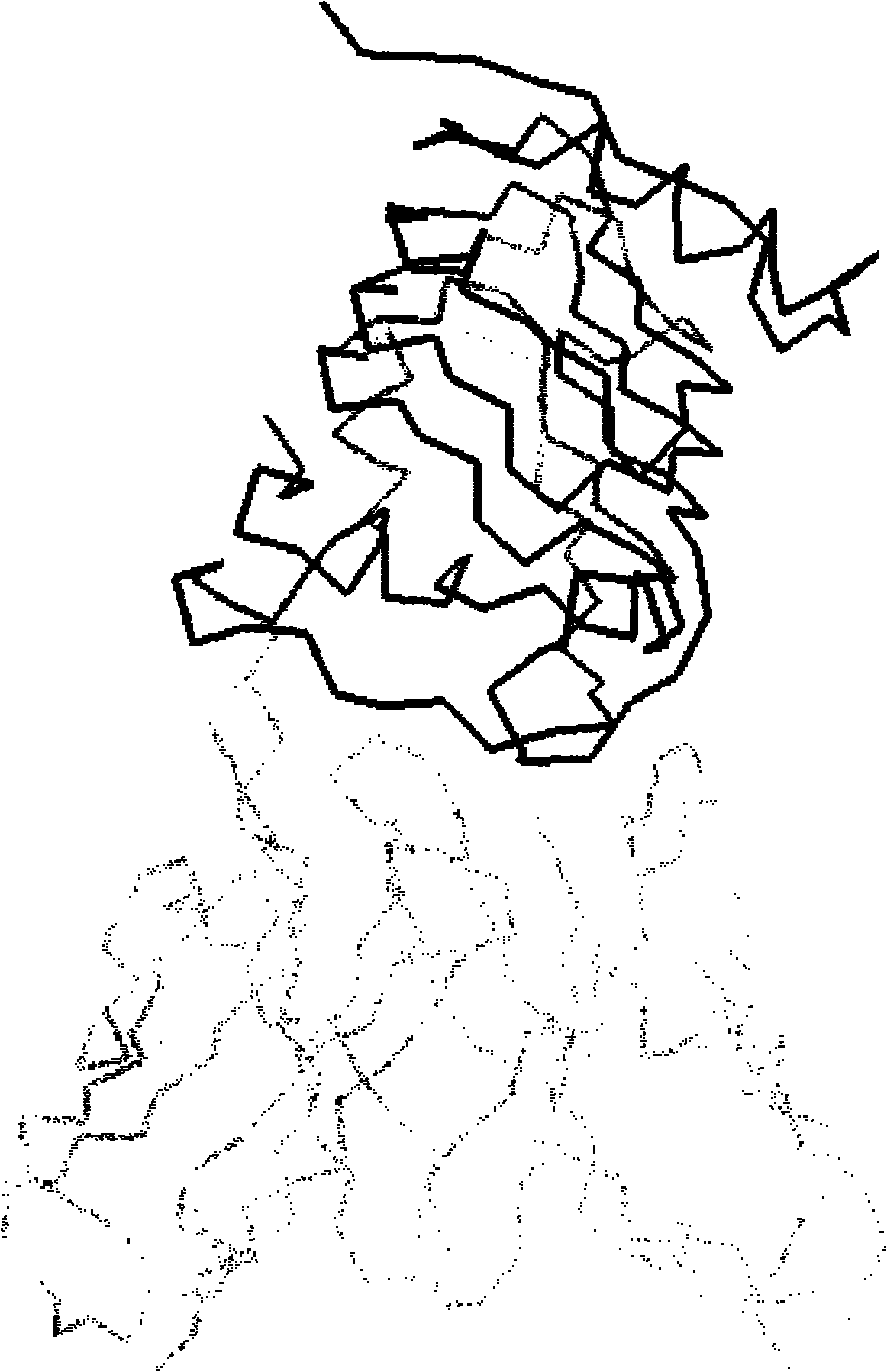
[0089] The sequence of the chimeric antibody chA21 has already been described by L.S.Cheng et al. (Chen L.S., Liu A.P., Liu.J. Construction, expression and characterization of the engineered antibody against tumor surface antigen P185 c-erbb-2 .Cell Res.2003, 13:35-48), such as figure 1 shown. Structure of chimeric antibody chA21: two identical protein chains joined by a disulfide bond formed in the Fc region. Each protein chain in turn consists of a light chain variable region (V L ), linker, heavy chain variable region (V H ) and the Fc region are sequentially connected, and its amino acid sequence is shown in SEQ ID NO: 9, wherein the 1-114th position is V L , the 115th-134th bit is linker, the 135th-253rd bit is V H , the 254th-489th position is Fc.
[0090] 1. Selection of Human Antibody Template
[0091] Sequence similarity comparison: compare the amino acid sequence similarity between the li...
Embodiment 2
[0114] Example 2. Construction of expression vectors for various antibodies
[0115] 1. Construction of pSectag2A-dFc vector
[0116] The pSectag2A vector was purchased from Invitrogen. Use PCR to amplify the Fc fragment, design primers to add EcoRI and XhoI restriction sites at the N-terminal and C-terminal of the Fc fragment, respectively, then double-digest the Fc fragment with EcoRI and XhoI, and recover the double-digested fragment; at the same time, pSectag2A plasmid Carry out double digestion with EcoRI and XhoI, and recover the double-digested fragment; connect the double-digested Fc fragment and pSectag2A vector with T4-DNA ligase, transform the ligated product into Top10 competent cells, and extract the plasmid for double-digestion. Restriction identification and sequencing verification, as a result, the gene sequence inserted between the EcoRI and XhoI restriction sites of pSectag2A is shown in nucleotides 760-1470 in SEQ ID NO: 2 (ie the Fc fragment), indicating t...
Embodiment 3
[0156] Example 3, Transient 293T cell expression of humanized antibody and purification of expression product
[0157] (1) Expression and purification of antibodies H1-1, H1-2, H2-1, H1-V1, ChA21
[0158] 293T cells were purchased from ATCC, catalog number CRL-11268.
[0159] DMEM medium was purchased from GIBCO, the product catalog number is 11960.
[0160] Cultivate a sufficient amount of 293T cells (DMEM / 10% serum / 1% double antibody) in advance (double antibody is penicillin and streptomycin, purchased from Shanghai Sangong, product number BS732), when the cells reach about 80% fullness, in the right Several growth phases were used to transfect recombinant antibody plasmid DNA.
[0161]The transfection steps are as follows: (1) Add 20ul Lipofectamine 2000 to 1ml DMEM, mix well with fingers or a pipette tip, and let stand at room temperature for 5 minutes. (2) Then add 10ug of plasmid DNA ready for transfection to 1ml DMEM and mix well. (3) Mix (1) and (2) (need to trans...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com