Method for synthesizing titanium silicon zeolite material
A technology of titanium silicalite and zeolite, which is applied in the field of synthetic titanium silicalite materials, can solve the problems of poor activity stability, easy deactivation, low activity of titanium silicalite, etc., and achieve increased selectivity of reaction products, reduced preparation costs, and good The effect of catalytic activity stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
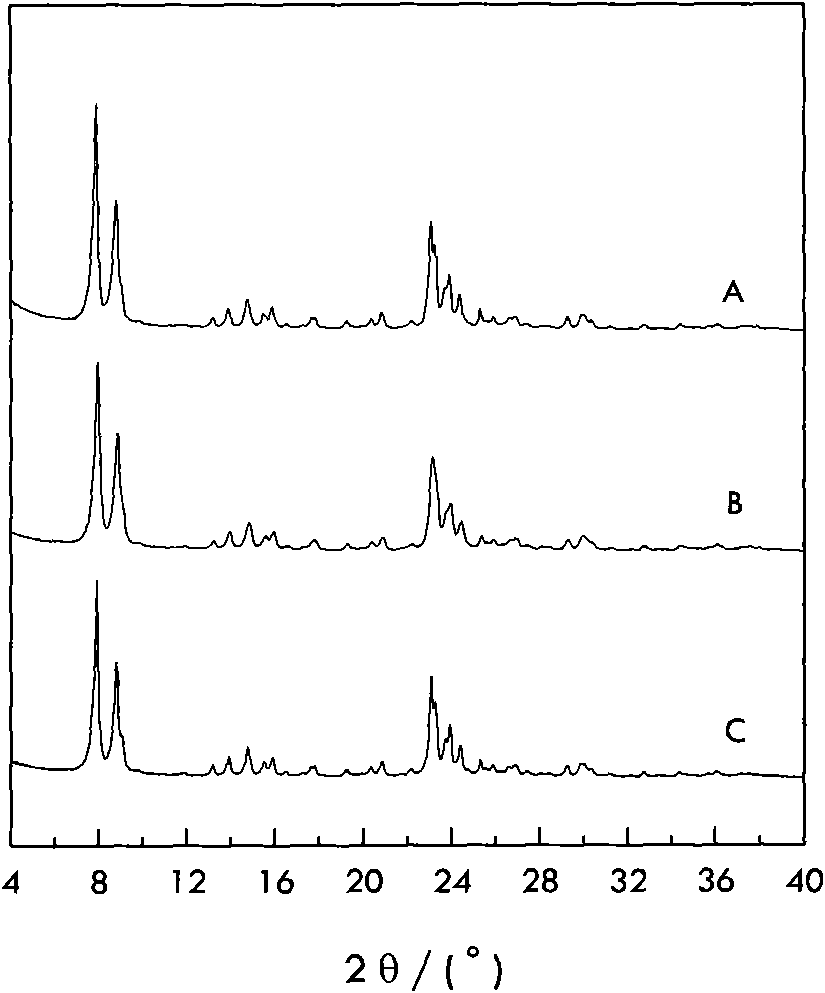
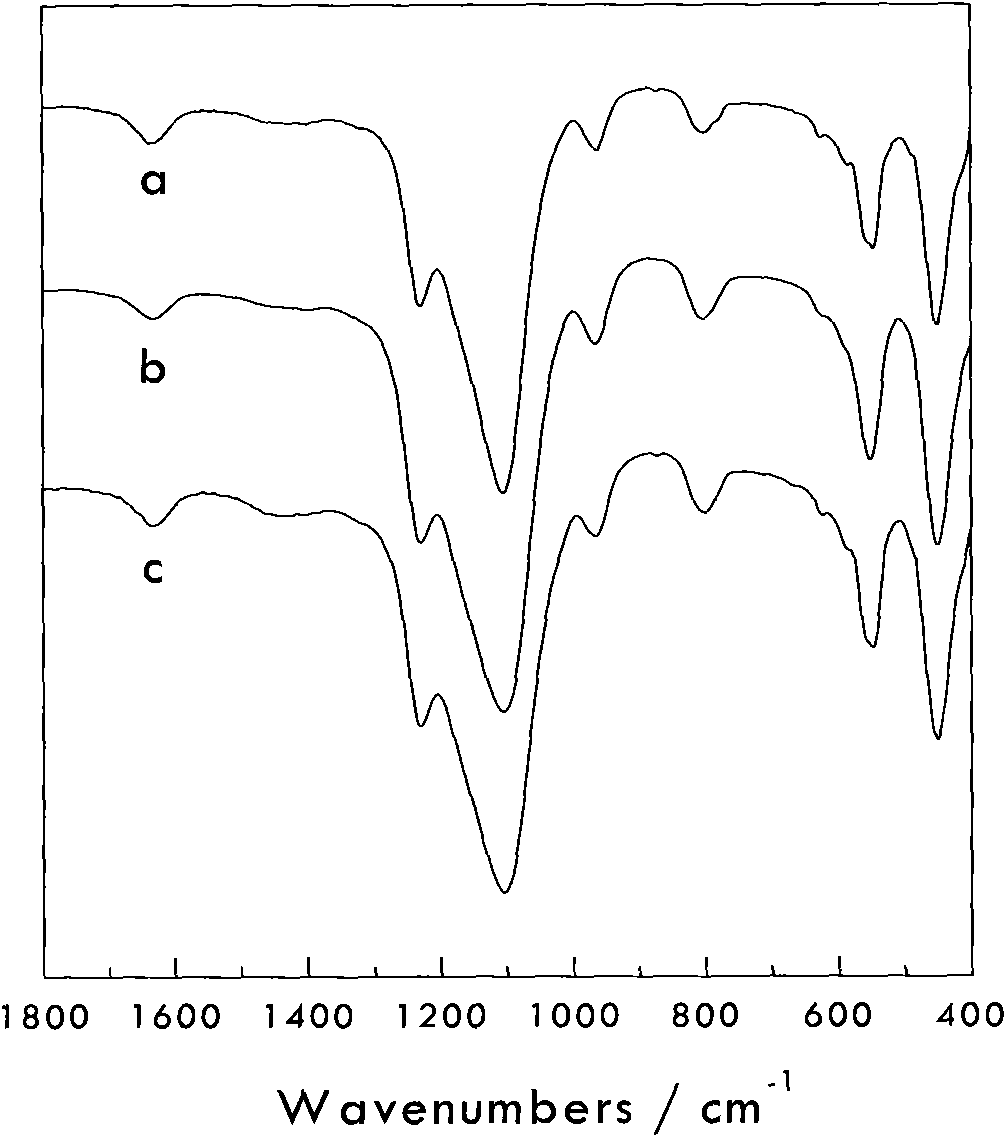
[0032]Add 20 grams of all silicalite S-1 into tetrapropyl ammonium hydroxide aqueous solution and stir to mix evenly, then add tetrabutyl titanate and mix well to obtain a mixture consisting of: all silicalite (gram): titanium source (mol ): alkali source (mol): water (gram)=100: 0.2: 0.2: 600. Then put it into a sealed stainless steel reaction kettle, hydrothermally treat it at 150°C and autogenous pressure for 48 hours, filter the resultant, wash with water, dry it naturally, and roast it at 550°C for 5 hours to obtain titanium silicalite material A. Its XRD crystal phase diagram is as follows figure 1 Shown in (B), similar to comparative example; Fourier transform infrared spectrogram is as figure 2 As shown in (b), it is also similar to the comparative example, at 960cm -1 The infrared absorption peak that all-silicon molecular sieves do not have appears nearby, indicating that titanium has entered the molecular sieve framework, and the 960cm -1 Absorption peak at and ...
Embodiment 2
[0034] Add 20 grams of all-silica zeolite S-1 into an aqueous solution of sodium hydroxide and stir to mix evenly, then add tetrapropyl titanate and mix well to obtain a mixture consisting of: all-silica zeolite (g): titanium source (mol): base Source (mole):water (gram)=100:5.0:1.2:3500. Then put it into a sealed stainless steel reaction kettle, hydrothermally treat it at 180°C and autogenous pressure for 24 hours, filter the resultant, wash with water, dry it naturally, and roast it at 550°C for 5 hours to obtain titanium silicalite material B. Its XRD crystal phase diagram is as follows figure 1 Shown in (C), similar to comparative example; Fourier transform infrared spectrogram is as figure 2 Shown in (c), also similar to the comparative example, in the infrared spectrum at 960cm -1 The infrared absorption peak that all-silicon molecular sieves do not have appears nearby, indicating that titanium has entered the molecular sieve frame, 960cm -1 Absorption peak at and 55...
Embodiment 3
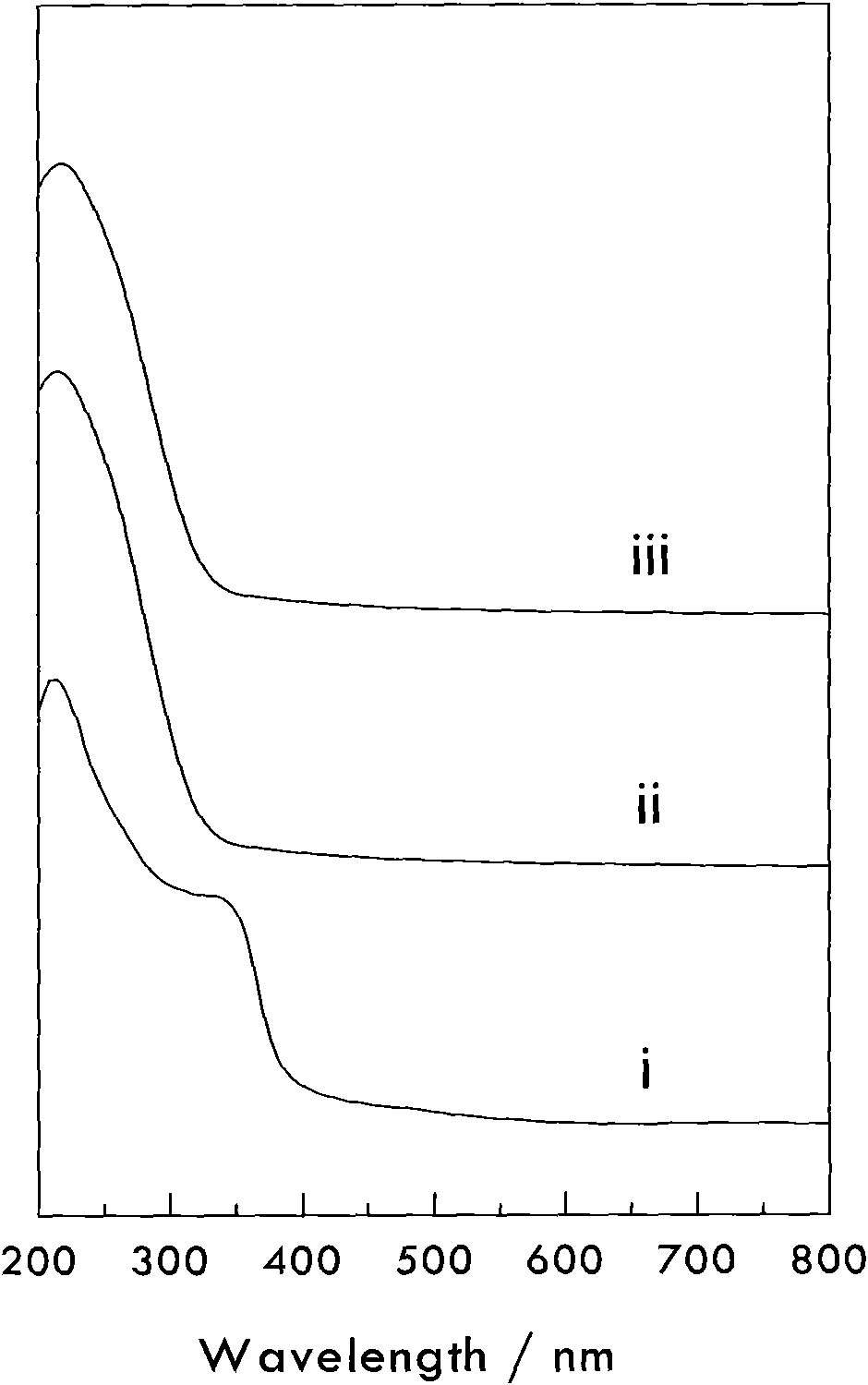
[0036] Add 20 grams of all silicalite S-1 into tetrapropyl ammonium hydroxide aqueous solution and stir to mix evenly, then add tetraethyl titanate and mix to obtain a mixture consisting of: all silicalite (gram): titanium source (mol ): alkali source (mol): water (gram)=100: 2.0: 1.0: 1200. Then put it into a sealed stainless steel reaction kettle, hydrothermally treat it at a temperature of 150°C and autogenous pressure for 48 hours, filter the resultant, wash with water, dry it naturally, and roast it at 550°C for 5 hours to obtain titanium silicalite material C. In its ultraviolet-visible spectrum, there is a strong absorption band near the wavelength of 210nm, but there is no absorption near 340nm, indicating that all titanium enters the skeleton, and no non-skeleton titanium is produced. Its infrared spectrum is 960cm -1 Absorption peak at and 550cm -1 Absorption peak intensity ratio I 960 / I 550 The data are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com