Detection method of ATP (Adenosine Triphosphate) content and ATP aptamer sensor
An aptamer sensor and detection method technology, which is applied to biochemical equipment and methods, microbial determination/inspection, instruments, etc., can solve problems such as high cost and complicated operation, and achieve the effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
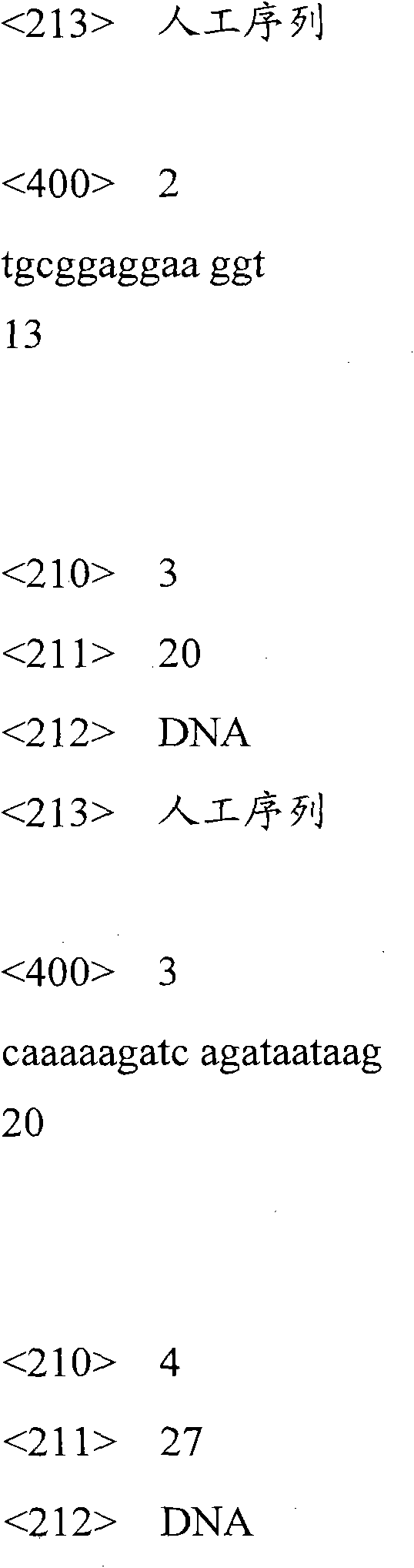
[0047] Divide the chain containing the ATP aptamer into two sequences, as shown in SEQ ID No.1 and SEQ ID No.2, which are the 3'end of the first strand in the single-stranded DNA and part of the double-stranded DNA;
[0048] The second strand in the partial DNA double strand is provided, as shown in SEQ ID No. 3, the second strand in the DNA double strand is complementary to the 5'end of the first strand in the partial DNA double strand, forming a partial DNA double strand (part ds -DNA).
Embodiment 2
[0050] After modifying the sulfhydryl group at the 5'end of the DNA single strand, the sequence 5'HS-(CH 2 ) 6 -ACCTGGGGGAGTAT-3’;
[0051] Dip the gold electrode (diameter 3mm) into the 5μM DNA single-stranded solution prepared in Example 1, and take it out after 1 hour;
[0052] After washing, the gold electrode is placed in a 10 mM mercaptohexanol (MCH) solution for 30 minutes;
[0053] Immerse the gold electrode placed in the mercaptohexanol (MCH) solution in the mixed solution of 5μM part ds-DNA and 5μM ATP prepared in Example 1, and take it out after reacting for 30 minutes;
[0054] Finally, put the gold electrode in 20mM Ru(phen) 3 2+ Reaction in solution for 1h.
[0055] ECL detection was carried out in 0.2M phosphate buffered saline (PBS) (pH 7.5, containing 20mM oxalate as a co-reactant), and cyclic voltammetry scanning was carried out in the range of 0-1.3 volts with a sweep rate of 0.05 volts / second.
Embodiment 3
[0057] After modifying the sulfhydryl group at the 5'end of the DNA single strand, immerse the gold electrode (3mm in diameter) in the 5μM DNA single strand solution prepared in Example 1, and take it out after 1 hour;
[0058] After washing, the gold electrode is placed in a 10 mM mercaptohexanol (MCH) solution for 30 minutes;
[0059] Dip the gold electrode placed in the mercaptohexanol (MCH) solution into the mixed solution of 5μM part ds-DNA and 10μM ATP prepared in Example 1, and take it out after reacting for 30 minutes;
[0060] Finally, put the gold electrode in 20mM Ru(phen) 3 2+ React in solution for 1h.
[0061] The ECL detection was performed in 0.2M PBS (pH 7.5, containing 20mM oxalate as a co-reactant), and the cyclic voltammetry scan was performed in the range of 0-1.3 volts, and the scanning rate was 0.05 volts / sec.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com