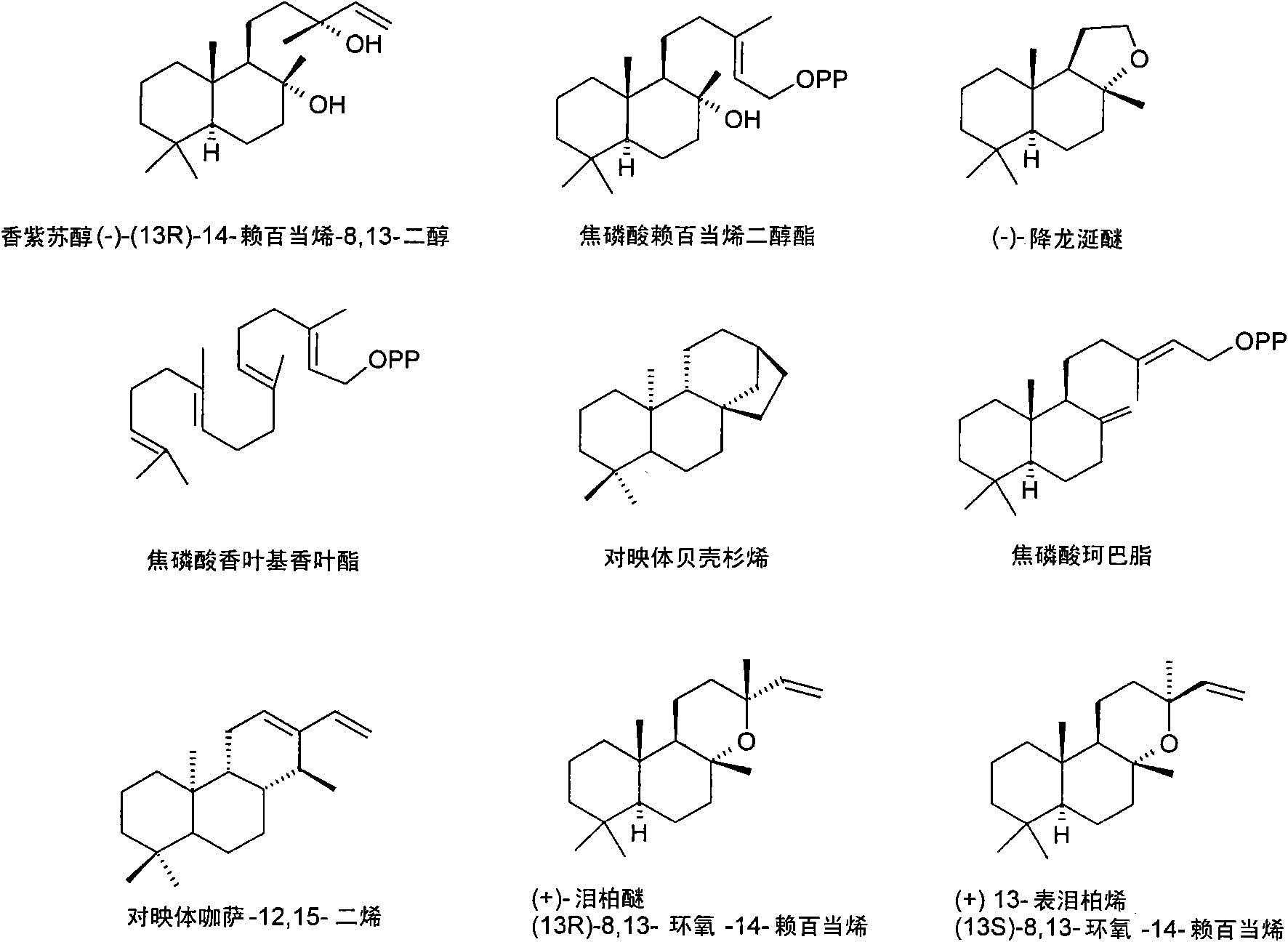
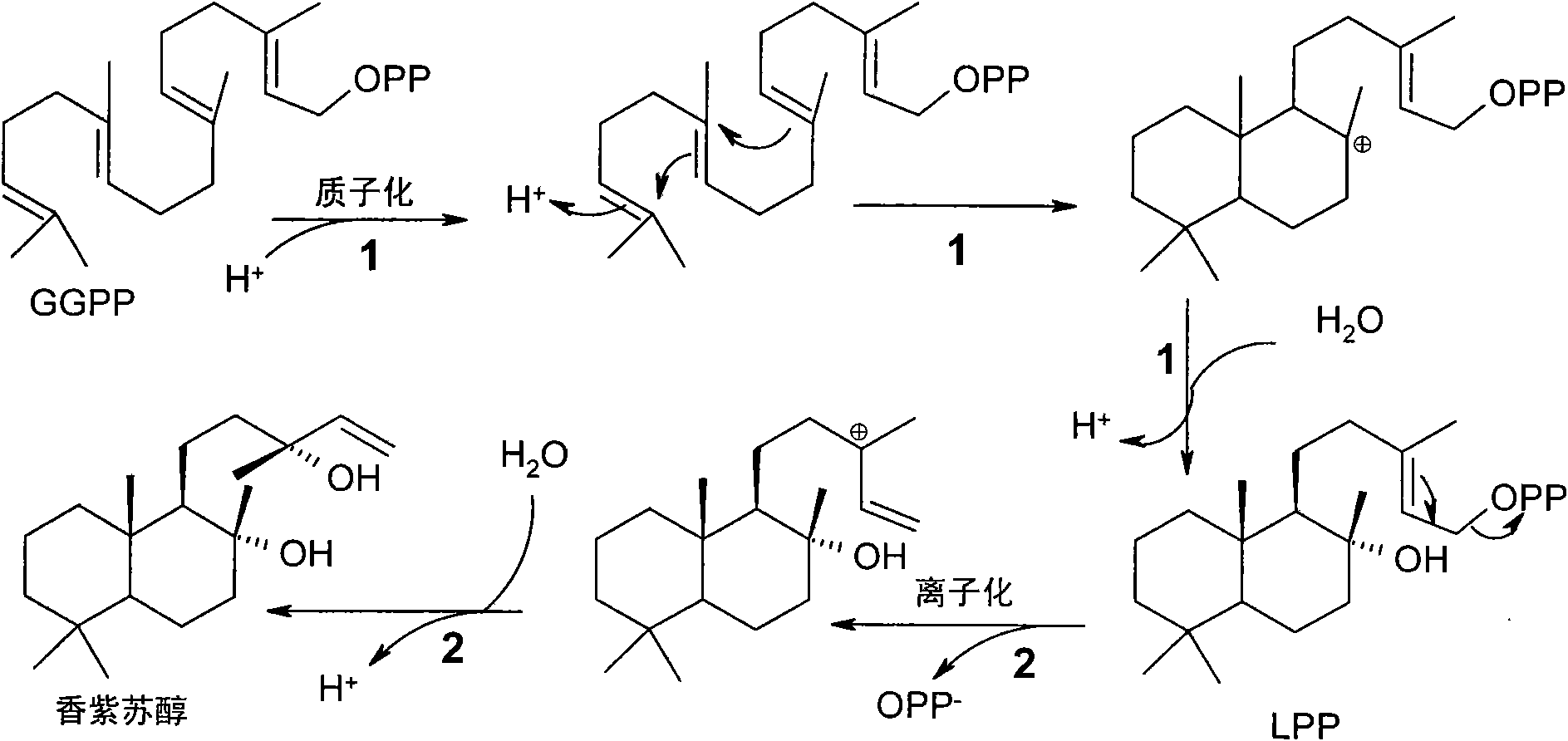
Method for producing sclareol
一种香紫苏醇、微生物的技术,应用在生物化学设备和方法、化学仪器和方法、酵素等方向,能够解决未公开香紫苏醇方法等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Isolation of LPP synthase encoding cDNA from Clary sage by PCR method
[0189] A. Plant Material and RNA Extraction
[0190] Clary sage with flower buds (1.5-2 cm long, 1-2 days old) were collected from the field in Bassins, Switzerland, and frozen directly in liquid nitrogen. Concert from Invitrogen (Carlsbad, CA) TM Total RNA was extracted with Plant RNA Reagent, according to the manufacturer's manual, with 2.0 mRNA Isolation Kit (Invitrogen, Carlsbad, CA) was used to purify mRNA by oligod T-cellulose affinity chromatography. Use Marathon TM cDNA Amplification Kit (Clontech, Mountain View, CA) was used to construct cDNA library.
[0191] B. Polymerase chain reaction for amplification of diterpene synthase cDNA
[0192] The amino acid sequences of class I and class II diterpene synthases from different plants were aligned and conserved motifs were selected. Degenerate oligonucleotide sequences were deduced from these conserved amino acid motifs. Designed usi...
Embodiment 2
[0198] Heterologous Expression of LPP Synthase from Clary Sage in Escherichia coli
[0199] pETDuet-1 (Novagen, Madison, WI), designed for expression under the control of the T7 promoter, was used for expression in E. coli cells. For construction of expression plasmids, forward and reverse primers SaTps-Nde (SEQ ID NO: 22) and SaTps designed to introduce an NdeI site immediately before the start codon and a KpnI site after the stop codon were used - Kpn (SEQ ID NO: 23), the open reading frame of SaTps1 (SEQ ID NO: 20) was amplified by PCR from a cDNA library. Since the reading frame contains an NdeI site at position 1614 of the reading frame, this amplification was performed in two steps by overlap extension PCR (Gene 77, 61-68, 1989 of Horton et al.), wherein primer SaTps-Nde( SEQ ID NO: 22) and SaTps-Kpn (SEQ ID NO: 23), together with primers Satps-mutlf (SEQ ID NO: 24) and Satps-mutlr (SEQ ID NO: 25), which were designed to remove the NdeI site and The amino acid sequen...
Embodiment 3
[0206] Purification and Enzyme Activity of LPP Synthase from Clary Sage
[0207] To further identify the recombinant diterpene synthase, we proceeded to purify SsLPPs3 and SsLPPs9 enzymes (SEQ ID NO: 1 and SEQ ID NO: 2).
[0208] The PCR2.1-Topo plasmid containing the SsLPPs3 cDNA and SsLPPs9 cDNA (SEQ ID NO: 4 and SEQ ID NO: 5) (Example 2) was digested with NdeI and SacI, and the insert was ligated into the pET28a(+) plasmid (Novagen) middle. The resulting expression plasmids (pET28-SsLPPs3 and pET28-SsLPPs9) contained cDNAs whose 5' end modifications (SEQ ID NO: 26 and SEQ ID NO: 27) were designed to express proteins with an N-terminal six-histidine tag . Purified under natural conditions using ProBond TM The purification system (Invitrogen) was performed according to the manufacturer's protocol except that, for elution, L-histidine was used instead of imidazole to minimize enzyme inhibition. Using this method, SsLPPs3 and SsLPPs9 recombinases can be purified to appare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com