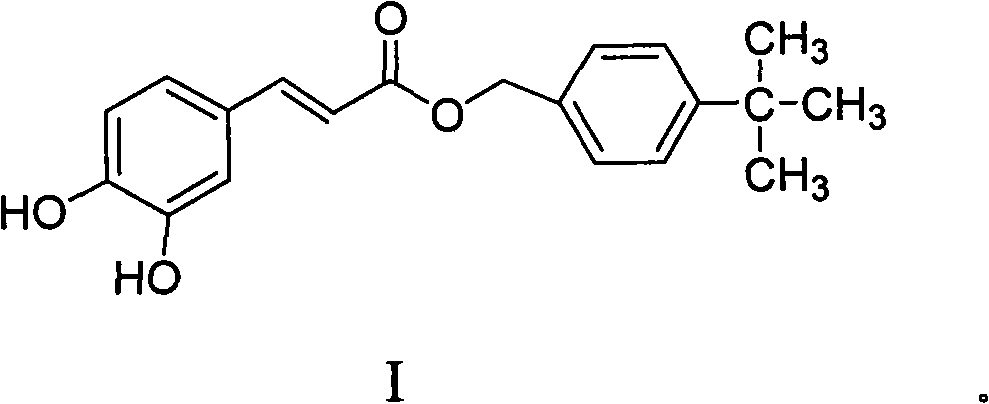
4-tertiary butyl benzyl-3,4-dyhydroxyl cinanmate as well as application and preparation method thereof
A use and technology of sodium carbonate, applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., can solve problems such as bad side effects, and achieve high yield, high clinical application value, and good development prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of 4-tert-butylbenzyl-3,4-dihydroxycinnamate:
[0037] Caffeic acid (5.0g, 27.8mmol) was added to the HMPA (60ml) solvent, fully stirred at room temperature, the solution was yellow and transparent, anhydrous sodium carbonate solid (3.5g, 33.4mmol) was added, stirring was continued at room temperature for 1 hour, and then A mixture of 4-tert-butylbenzyl bromide (5.6ml, 30.5mmol) dissolved in HMPA (10ml) was slowly dropped into the above reaction solution. Control the temperature at 20°C, stir and react for 20 hours. After TLC confirms that the reaction is complete, pour the reaction solution into 100ml of ice water, add 1N HCl to acidify to pH 6, extract three times with ethyl acetate, combine the organic phases, and successively use 1N HCl40ml and saturated aqueous sodium chloride solution, and the organic phase was dried over anhydrous magnesium sulfate for 3 hours. The solvent was evaporated under reduced pressure, separated and purified on a silica gel ...
Embodiment 2
[0045] Inhibitory effect of 4-tert-butylbenzyl-3,4-dihydroxycinnamate on glutamate-induced death of primary cultured cerebellar granule neurons in rats
[0046] Method and steps:
[0047] Firstly, the primary culture of cerebellar granule neurons (CGN) was carried out. After culturing for 7 days, 4-tert-butylbenzyl-3,4-dihydroxycinnamate (PAR-007, with a final concentration of 10 μM) and caffeic acid phenylethyl ester (CAPE, with a final concentration of 10 μM). Then, neurotoxin glutamate (Glutamate, final concentration 30 μM) was added to the cell culture medium, and the cells were placed in CO 2 Incubator (37°C, 5% CO 2 ) for 24 hours, and finally the cell viability was detected by the FDA / PI double staine method. The results show (see figure 1 ), 30μM glutamic acid (Glutamate) can induce significant death of CGN neurons, while 4-tert-butylbenzyl-3,4-dihydroxycinnamate (PAR-007, final concentration of 10μM) can significantly inhibit The death of neurons significantly p...
Embodiment 3
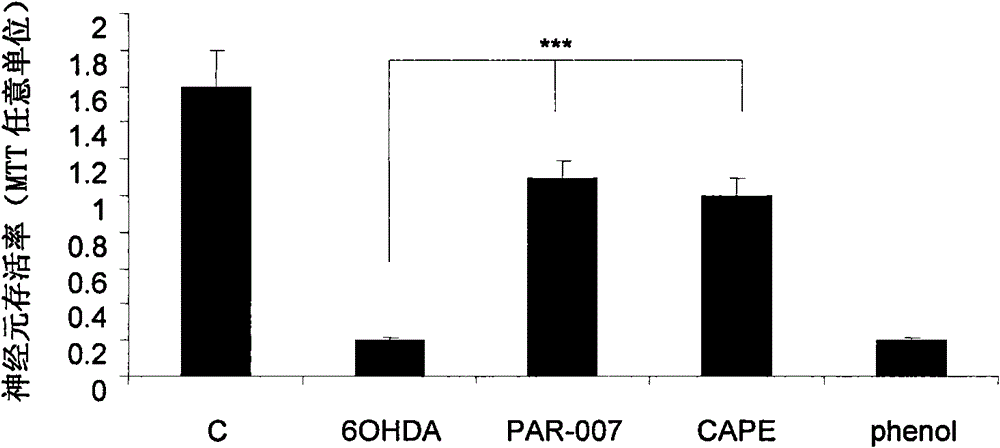
[0049] Inhibitory effect of 4-tert-butylbenzyl-3,4-dihydroxycinnamate on 6-hydroxydopamine (6-OH-DA)-induced death of primary cultured midbrain dopamine neurons in rats
[0050] Primary cultures of midbrain dopamine neurons were first performed. After culturing for 7 days, 4-tert-butylbenzyl-3,4-dihydroxycinnamate (PAR-007, with a final concentration of 10 μM) and caffeic acid phenylethyl ester (CAPE, with a final concentration of 10 μM). Then, neurotoxin 6-hydroxydopamine (6-hydroxydopamine, final concentration 50 μM) was added to the cell culture medium, and the cells were placed in CO 2 Incubator (37°C, 5% CO 2 ) for 24 hours, and finally the cell viability was detected by MTT assay. The results show (see figure 2 ), 50μM 6-hydroxydopamine can induce significant death of midbrain dopaminergic neurons, while 4-tert-butylbenzyl-3,4-dihydroxycinnamate (PAR-007, final concentration of 10μM) can significantly inhibit The death of neurons significantly protects nerve cells....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com