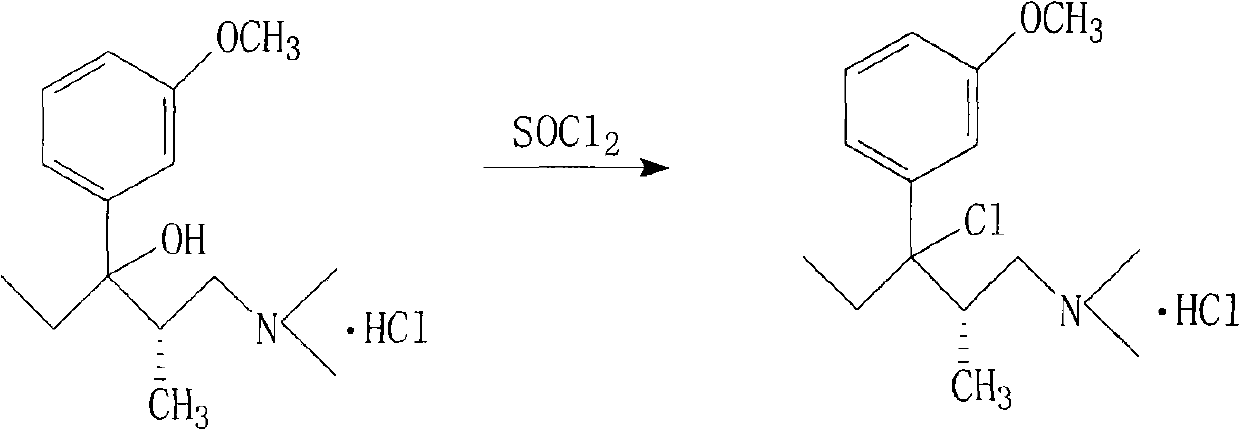
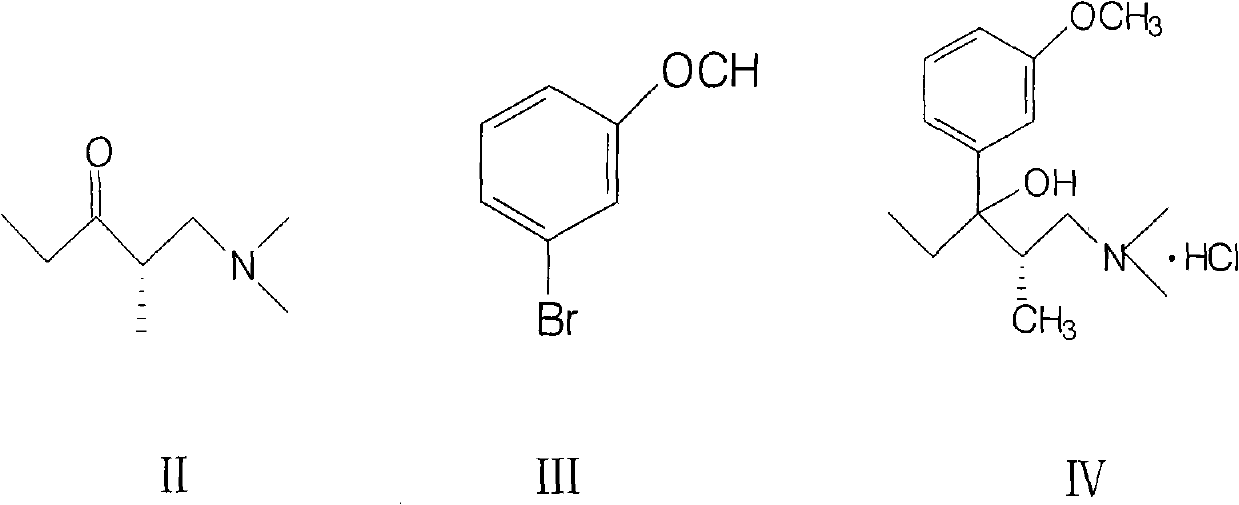
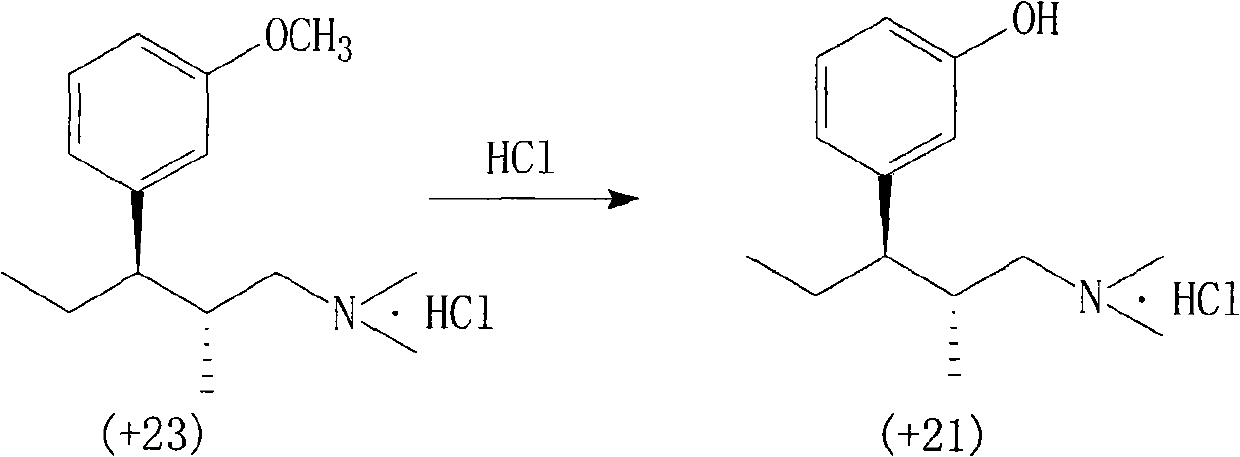
Method for preparing important intermediate of tapentadol hydrochloride analgesic
A tapentadol and intermediate technology, applied in the field of drug synthesis, can solve the problems of undesired cost economy and achieve the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0025] Preparation of (2S)-1-dimethylamino-3-(3-methoxyphenyl)-2-methylpentan-3-ol hydrochloride
[0026]
[0027] Add 26.99 grams (1.11 moles) of magnesium chips and 105 milliliters of anhydrous tetrahydrofuran into a dry and anhydrous four-necked flask, and add dropwise 207.63 g (1.11 moles) of 3-bromoanisole dissolved in 400 milliliters of anhydrous tetrahydrofuran under stirring solution, the reaction is exothermic, and the reaction solution is slowly boiled. After the addition of the 3-bromoanisole solution was complete, the mixture was heated to reflux for 1 hour and then cooled to 5-10°C. A solution of 128.3 g (0.89 mol) of (S)-1-dimethylamino-2-methyl-3-pentanone dissolved in 400 ml of tetrahydrofuran was added at this temperature. The reaction solution was allowed to stand overnight at room temperature, and then cooled to 5-10°C. Ammonium chloride solution was added to decompose Grignard's solution. The reaction mixture was diluted with ethyl acetate, the organi...
preparation Embodiment 2
[0029] Preparation of (2S)-1-dimethylamino-3-(3-methoxyphenyl)-2-methylpentan-3-ol hydrochloride
[0030]
[0031]Add 26.99 grams (1.11 moles) of magnesium chips and 105 milliliters of anhydrous tetrahydrofuran into a dry and anhydrous four-necked flask, and add dropwise 280.6 grams (1.5 moles) of 3-bromoanisole dissolved in 400 milliliters of anhydrous tetrahydrofuran under stirring solution, the reaction is exothermic, and the reaction solution is slowly boiled. After the addition of the 3-bromoanisole solution was complete, the mixture was heated to reflux for 1 hour and then cooled to 5-10°C. A solution of 114.6 g (0.8 mol) of (S)-1-dimethylamino-2-methyl-3-pentanone dissolved in 400 ml of tetrahydrofuran was added at this temperature. The reaction solution was allowed to stand overnight at room temperature, and then cooled to 5-10°C. Ammonium chloride solution was added to decompose Grignard's solution. The reaction mixture was diluted with ethyl acetate, the organi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com