Polypeptide WA8 for inhibiting type-2 shiga toxin activity and coding gene and application thereof
A Shiga toxin, coding gene technology, applied to peptides, peptide/protein components, recombinant DNA technology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
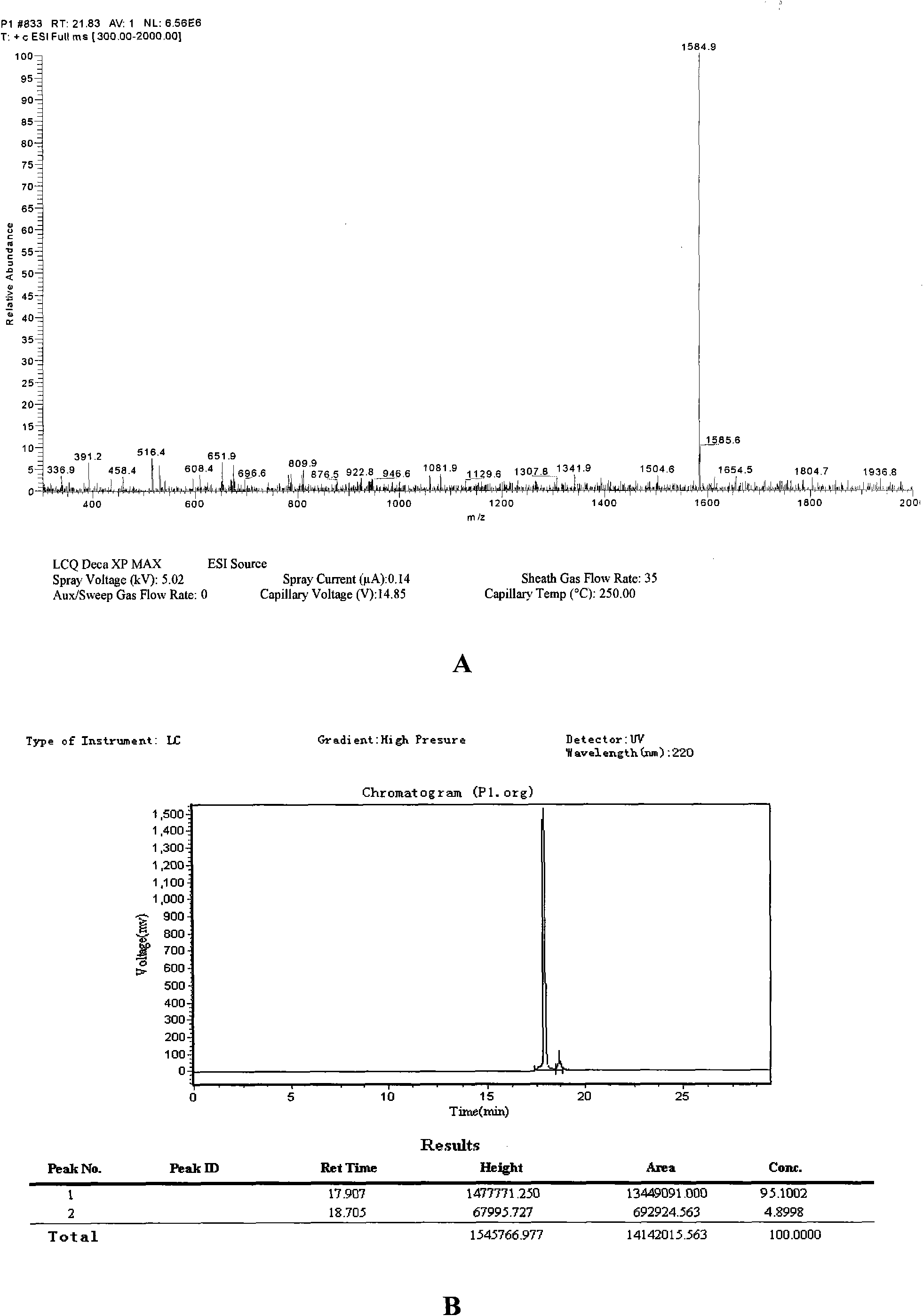
[0026] Example 1. Modification, chemical synthesis and binding activity analysis of short peptides TF1 (also known as P1) and WA8 (also known as P8)
[0027] 1. Modification and synthesis of short peptides TF1 (also known as P1) and WA8 (also known as P8)
[0028] 1. Screening of TF1 and WA8 synthetic peptide sequences
[0029] Preparation of Stx2B protein: using EHEC O157:H7 bacteria (purchased from ATCC) as a template, the stx2B gene was amplified with primers 5'-catatggctaaaggtaaaattgag-3' and 5'gcggccgcgcctcagtcatcagtcatt-3'. The pEASY-T1 clone vector was used for sequencing identification, and the sequencing results showed that the nucleotide sequence of the amplified product was as the 1353327th to 1353533rd positions of GenbankNC_002655. The amplified product was inserted into the Nde I and Not I restriction sites of the pET22b+ vector (purchased from Novagen) to form the recombinant plasmid pET22b-stx2B, and then pET22b-stx2B was introduced into the E.coli.BL21 (DE3) st...
Embodiment 2
[0043] Example 2. In vitro cytotoxicity inhibitory activity of TF1 and WA8 synthetic peptides and binding blocking effect on target cells of toxins
[0044] HeLa cell line, trypsin digestion solution and double antibody were purchased from Hyclone Company, DMEM medium was purchased from GIBCO Company, fetal bovine serum was purchased from Biochorm Company, MTT was from Sigma Company, and FITC was purchased from Leybold Company. Cell culture flasks and culture plates were purchased from CORNING Company, CO 2The incubator is a product of SANYO Company of Japan, the inverted optical microscope is a product of Olympus Company of Japan, and the flow cytometer is a product of Coulter Company.
[0045] 1. Cytotoxicity neutralization experiment
[0046] Collect the cultured logarithmic phase cells (HeLa cells), adjust the concentration of the cell suspension, add 100 μl to each well, and plate to adjust the density of the cells to be tested to 10 4 / well (edge wells filled with st...
Embodiment 3
[0049] Example 3. Protective effect of TF1, WA8 synthetic peptides on type 2 Shiga toxin-challenged mice
[0050] Male Balb / C (17-19g) mice were provided by the Animal Center of Academy of Military Medical Sciences. Test polypeptide TF1 (also known as P1) and WA8 (also known as P8) purity> 95% (g grade)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com