Application of eucheuma gelatinae polysaccharide to preparation of medicines for inhibiting respiratory viruses
An anti-respiratory and Eucheuma technology, applied in the field of medicine, can solve problems such as impact and complex anti-coagulation effects, and achieve high promotional value, low toxicity, and strong anti-respiratory virus activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, Determination of Molecular Weight Range of Eucheuma Polysaccharide
[0024] a. Precisely weigh 1mg of Eucheuma polysaccharide, dissolve it in 0.2M sodium acetate buffer solution with a pH value of 4.0 to a final concentration of 1mg / ml, and filter it through a 0.45um microporous membrane for use.
[0025] b. Dextran Mw800T, 505T, 170T, 60T. Accurately weigh 0.100 g of dextran standard substances with different molecular weights, dissolve them in mobile phase and dilute to 10 mL, and the final concentration is 10 mg / mL.
[0026] c. The standard product and Eucheuma polysaccharide samples were detected by high performance liquid phase, the mobile phase was 0.2M sodium acetate buffer solution with a pH value of 4.0, the chromatographic conditions were flow rate 1.0mL / min, injection volume 10μl, injection time 15min . The coordinate curve was drawn with the separation retention time (RT) of different molecular weight standard dextran and the corresponding ...
Embodiment 2
[0028] Embodiment 2, Study on Cytotoxicity of Polysaccharides from Eucheuma agaricus
[0029] 1. Test material
[0030] The extracted Eucheuma polysaccharide was prepared into a 10 mg / mL aqueous solution with pure water, and stored at -20°C for later use.
[0031] 2. Test method
[0032] Dilute Eucheuma agaricus polysaccharides to different concentrations (initial concentration is 500μg / mL), and observe under an inverted microscope to observe the maximum non-toxic concentration of the drug TC 0 , Determination of cytotoxicity of Eucheuma polysaccharides.
[0033] 3. Test results
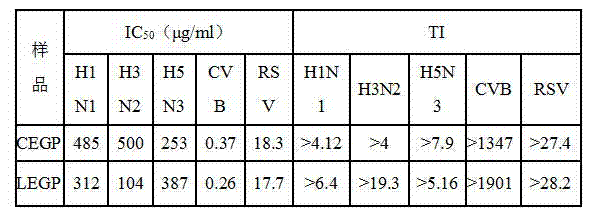
[0034] The highest non-toxic concentration TC0 for each strain of cells is taken as the highest drug dilution concentration that does not cause cell death in normal cells and does not cause cytopathic changes. LEGP) components were not toxic to MDCK, HeLa, Hep-2 cells at the concentrations used in the experiments.
[0035] Table 1 Cytotoxicity of CEGP and LEGP
[0036]
Embodiment 3
[0037] Embodiment 3, Anti-respiratory tract-associated virus efficacy experiment of Eucheuma qiongzhi polysaccharides
[0038] a. Polysaccharides were extracted from Eucheuma agaricus, with an average molecular weight of 45,000 and a monosaccharide sulfate group substitution degree of 2.13.
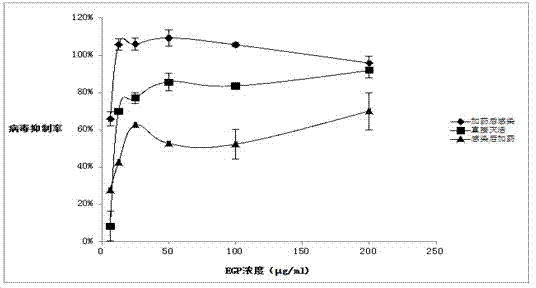
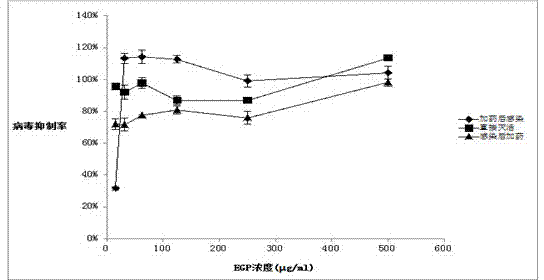
[0039] b. Referring to the results of the cytotoxicity experiment in Example 2, the Eucheuma agaricus polysaccharide extracted in step a was prepared into a dilution with an initial concentration of 62.5 μg / mL, and the anti-respiratory tract-associated virus activity was determined by the MTT method.
[0040] c. Step: Inoculate cells in 96-well culture plates with a cell density of 1.5*10 5 pcs / ml, 100μl per well, 37℃, 5%CO 2 Cultivate in the incubator for 16-20 h, wait until it grows into a single layer of cells, discard the culture medium, wash with PBS three times, and mix different dilutions of drugs (within the non-toxic concentration range) and virus dilution (100TCID 50 ) each 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com